This publication provides a glossary of botanical terms related to hazelnut pollination and describes the flower and nut development process, pollination, and related issues
Parts of Florets (female)
Stigma. Portion of the style that is receptive to pollen germination. The stigmatic surface of hazelnut female flowers is red and covers the entire surface of the style.
Style. Elongated stalk that connects the stigma to the ovary.
Ovary. Basal portion of the female flower that bears the ovules. The ovary consists of a wall of tissue (eventually the nut shell) and two ovules.
Ovule. Structure within the ovary that bears the egg.
Egg. Female cell that (after fertilization) develops into the embryo.
Parts of Catkins (male)
Anther. Pollen-bearing portion of the stamen.
Catkin. Pollen-producing organ.
Pollen. Grains bearing sperm formed in anthers on catkins.
Pollen germination. Growth of a pollen tube out of the pollen grain. This occurs when pollen is placed under suitable conditions, such as on the stigmatic surface of compatible flowers.
Pollen tube. Tube bearing the sperm. In the process of germination, the tube grows down the style and into the ovary, where it transfers the sperm to the egg.
Pollination. Transfer of pollen from the anther of the male flower to the stigma of a female flower.
Pollinizer. Producer of pollen; the variety used as a source of pollen for cross-pollination.
Fertilization. Union of sperm and egg to start the new generation (embryo or kernel).
Nut, Kernel, and Flower Development
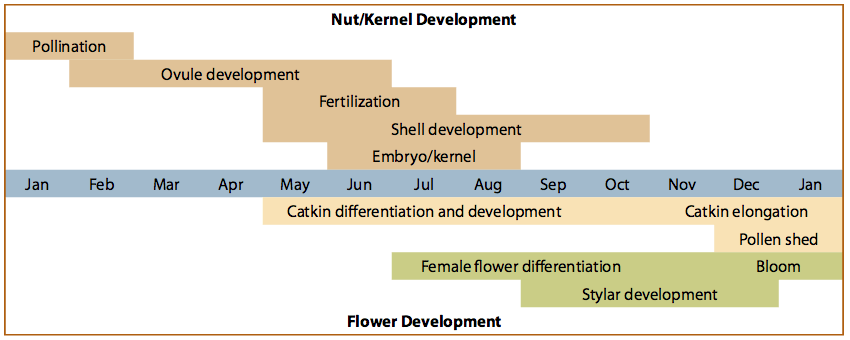
The life of a hazelnut begins with the formation of flower clusters more than a year before harvest (Figure 1). During fruit bud differentiation, a cell forms either a reproductive part, such as a flower, or a vegetative part, such as a shoot.
Male catkins (Figure 2) begin to form in mid-May and begin to appear in June, but they do not reach maturity until December or January.
Female flower parts (Figure 3) begin to form at the end of June and beginning of July and are first seen in late November or early December. Most flowers form on the current season’s growth in the axils (where leaves join the stems). On some varieties, flowers also form on the peduncles (stems) of the catkins.
Peak pollination season is from January through February, but this can vary depending on weather conditions. During the pollination season, the female cluster is a bright red tuft of feathery stigmatic styles projecting out of the bud scales. Within the bud scales are the lower portions of 4–16 individual flowers.
Female hazelnut flowers are very unusual. At pollination, most plants’ flowers have an ovary that contains ovules with egg cells ready for fertilization. Hazelnut flowers, however, have several pairs of long styles with surfaces that are stigmatic and receptive to pollen, and a tiny bit of tissue (0.25 mm or less) at the base called the ovarian meristem.
Within 4–7 days after pollen transfer (pollination), the pollen tube grows to the base of the style, where the tip of the tube bearing the sperm becomes “walled off ” and enters a long resting period. Pollination stimulates the ovary to develop from the tiny meristematic tissue at the base of the flower. The ovary grows very slowly the first 4 months (until about mid-May) and then begins to grow rapidly, attaining 90% of its growth in the next 5–6 weeks. During the middle of this rapid growth period (mid-June), when the ovaries are 8–10 mm in diameter, the ovary becomes a mature organ containing egg cells.
The resting sperm become activated, secondary pollen tubes begin to grow, and fertilization takes place. This 4- to 5-month lapse between pollination and fertilization is one of the unusual features of hazelnut floral biology. In most other plants, fertilization follows pollination by a few days.
After fertilization, the kernel develops rapidly. By mid-July, the ovary’s shell is full size, and shell hardening is well under way. Kernels reach full size about 6 weeks after fertilization (early August). From this time until harvest, maturation changes occur. For example, oil content increases. Around mid-September to October, depending on the variety, the husk surrounding the nut dries and spreads open, and the nut falls to the ground.
Pollination
Hazelnuts are monoecious, meaning they have separate male and female flowers on the same tree. Male and female flowers may bloom at different times. Hazelnuts are self-incompatible, which means a tree cannot set nuts with its own pollen. Also, certain combinations of varieties are cross-incompatible. That is, pollen of some varieties will not set nuts on certain other varieties.
A single gene, located at a specific position on the chromosome, controls this type of incompatibility. There are more than 30 known alleles (forms) of this gene, each of which is identified by a number. Because hazelnuts have two sets of chromosomes, they have two alleles for this gene—one on each of two chromosomes. These alleles are identified as SxSy. For example, the alleles for the variety Lewis are S3S8. In the female flower, alleles on both chromosomes are expressed. The pollen from the male flower may express one or both alleles. If an expressed allele in the pollen matches either of the alleles in the female flower, the cross is incompatible.
Hazelnut trees are wind pollinated, and there must be a compatible pollinizer variety for effective pollination. Additionally, the time of bloom for male and female flowers is important because receptivity of the female flowers must overlap with the time of pollen shed.
We recommend placing three pollinizer varieties (early, mid, and late) in an orchard so pollen is available throughout the extended period of time during which female flowers appear. Stigmas are receptive to pollen from the time they first appear as a tiny red dot at the tip of the bud until they extend to their maximum length. In some seasons, this occurs from late November until early March.
Pollinizer Spacing and Placement
Each catkin produces more than 1 million functional pollen grains, and each tree usually bears several thousand catkins. The quantity of pollen usually is not a factor in determining the number of pollinizer trees needed, but the placement of pollinizer trees in the orchard can be.
The wind can carry pollen grains great distances, but the density of the pollen cloud decreases with the distance from the source tree. Thus, distance is the most important consideration in pollinizer placement. To take best advantage of the wind, disperse pollinizer trees throughout the orchard rather than plant them in solid rows. Some evidence suggests that nut set decreases when the distance between pollinizers and recipient trees is more than 50 feet (15 meters).
Standard pollinizer placement is every third tree in every third row (3 × 3) for a standard, low-density orchard planted at 20 × 20-foot spacing. In this arrangement, about 11% of the trees are pollinizers, and the pollinizers are within the recommended distance range of recipient trees.
Blanks, Brown Stain, and Flower Cluster Loss
Anything that prevents normal nut development reduces yield. The three most common problems are blanks, brown stain, and the early drop of female flower clusters.
Blanks
Blank nuts (a shell without a kernel) reduce the yield potential of all varieties. Blanks occur when pollination stimulates the shell to develop but the kernel fails to develop normally. The kernel either fails to grow at all or starts to grow and then aborts, often in the early stages of growth. Some kernels might grow to more than half their expected size and then shrivel. Processors cull out these small kernels along with blanks.
Lack of pollination is not the cause. If pollination had failed, the shell would not have developed at all. Factors that contribute to a high percentage of blank nuts are not definitely established. Research suggests the following possibilities: insufficient soil moisture in midsummer, low light levels in overgrown orchards, tendency of main tree variety, pollinizer variety, and inadequate tree nutrition (particularly nitrogen, boron, and potassium).
Brown Stain
Brown stain (Figure 4) is a disorder of unknown cause that can result in severe crop loss in some seasons. Brown stains are seen on the sides of nuts in early summer. Affected nut clusters often drop from the tree in July and August. Many affected nuts are blanks or only partially filled. Among the main commercial varieties, only Barcelona has a serious brown stain problem.
Flower Cluster Loss
Early drop of female clusters is even more serious than blanks. In Barcelona, 35%–50% of clusters produced on a tree can drop prematurely. The potential loss from these “developmental dropouts” amounts to 75%–85% of the total individual flowers produced by the tree.
The majority of flowers in these clusters have been pollinated, and development has progressed for a few months at the normal slow rate before growth is arrested. Although the individual flowers are larger (1–2 mm diameter) at their base than at the time of pollination (0.25 mm), the clusters are still so small that their dropping is not conspicuous in the orchard. Some of the flowers in clusters that do not drop from the tree (because at least one nut has developed normally) also are subject to arrested growth. Close examination of nut clusters during summer can reveal a few tiny, undeveloped flowers embedded among the fleshy husks.
Foliar-applied boron can increase nut cluster set by as much as 33%. Use a single spray of Solubor (sodium pentaborate) around mid-May. Do not apply more than 1 lb of actual boron (4.88 lb Solubor) per acre (1.1 kg actual boron or 5.48 kg Solubor per hectare) because excess boron can be toxic (Figure 5).You can make annual applications of foliar boron until leaf boron concentrations are 200 ppm. Then skip a year or two of foliar boron.
For More Information
Many Oregon State University Extension publications on hazelnut production are available through the OSU Extension Catalog:
This information is provided for educational purposes only. If you need legal [or tax] advice, please consult a qualified legal [or tax] adviser.
Trade-name products and services are mentioned as illustrations only. This does not mean that the Oregon State University Extension Service either endorses these products and services or intends to discriminate against products and services not mentioned.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.
© 2018 Oregon State University.
Extension work is a cooperative program of Oregon State University, the U.S. Department of Agriculture, and Oregon counties. Oregon State University Extension Service offers educational programs, activities, and materials without discrimination on the basis of race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, familial/parental status, income derived from a public assistance program, political beliefs, genetic information, veteran’s status, reprisal or retaliation for prior civil rights activity. (Not all prohibited bases apply to all programs.) Oregon State University Extension Service is an AA/EOE/Veterans/Disabled.