The Pacific Northwest hazelnut industry has a long history of using integrated pest management to manage insect, disease, and vertebrate pests. IPM involves the carefully managed use of an array of pest control tactics—including biological, cultural, and chemical methods—to achieve the best results with the least environmental disruption. This approach helps growers avoid unnecessary sprays and make timely applications when truly needed. The investment in developing a hazelnut IPM program has paid off, and economic and environmental benefits continue to accrue.
This publication provides an overview of insect, disease, and vertebrate pest management in hazelnuts. For specific, current management recommendations, see Hazelnut Pest Management Guide for the Willamette Valley and the current editions of the Pacific Northwest Insect Management Handbook and Pacific Northwest Plant Disease Management Handbook. Products registered for use change from year to year, and these publications are updated regularly.
Insect management
Several insect pests infest hazelnut orchards. The most significant are filbertworm, aphids, and leafrollers. Management of these pests involves monitoring pest populations to time pesticide applications most effectively. You can monitor some pests by direct observation of pests on the plant. For other pests, you can use traps baited with pheromones (sex hormones). There is an action threshold—the level of infestation that triggers control measures—for each pest (Table 1).
Filbertworm
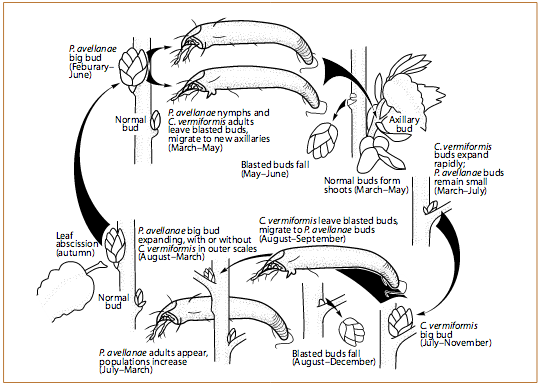
Filbertworm (Melissopus latiferreanus) is the most significant insect pest in hazelnut orchards. It damages nuts by feeding on the developing kernel. Its life cycle is unusual, in that the emergence period for a single generation extends from late June through early October. This extended emergence period makes the use of pheromone traps vital for determining the most effective timing for insecticide applications. Occasionally, a second generation of filbertworm emerges, but the populations and pest pressure are lower than for the earlier generation. This generation usually is 10% of the population size of the first generation. The second generation often occurs when there is a strong, early emergence of the first generation.
The filbertworm overwinters as an inactive larva and, eventually, pupa in a silken cocoon that loosely incorporates twigs and leaf material from the orchard floor (Figure 1). Cocoons can be found under leaves and trash on the orchard floor or in any available crack or crevice. Some larvae also hibernate 1–2 inches (2.6–5.1 cm) beneath the soil surface.
Larvae pupate in the cocoon in the spring and begin emerging as adults about 2–5 weeks later (Figure 2). This emergence usually begins around mid-June and continues through October. When the adults emerge, they look for mates. Favorable conditions for mating flights and egg laying are when temperatures at dusk (from 30 minutes before to 2 hours after sunset) are higher than 60°F (15.7°C).
The day after mating, egg laying starts. Eggs are usually laid during the sunniest part of the day. Eggs are laid one by one on leaves within 6 inches of nut clusters and hatch within 8–10 days, depending on temperatures. The newly hatched larvae then move to find a nut to feed in (Figure 3). They will feed in the nut for about 2–4 weeks. Then they either enlarge their entry hole to exit the nut, or bore a new exit hole.
Action thresholds for filbertworm (Table 1) are based on four pheromone traps for the first 10 acres and one trap for each additional 4 acres. When monitoring, place pheromone traps in the top third of the tree canopy (unlike leafroller traps, which are placed at eye level). Store extra pheromone caps in a refrigerator or freezer, and replace the pheromone dispensers every 4–6 weeks. Change the trap bottoms when soil and insects have accumulated enough to prevent catches or easy counting.
Be aware of the conditions around your orchard. Sometimes pregnant females from outside areas fly into uninfested orchards and lay eggs. If an abandoned or heavily infested block is within half a mile of your orchard, place pheromone traps in the part of your orchard that is closest to it and check them regularly. Oak trees are the primary host for filbertworm.
Spot spraying is successful in specific situations. When traps in the orchard’s border show a strong flight of moths, spray at least four to six outside rows that are nearest to the abandoned or heavily infested areas.
Filbert and hazelnut aphids
Aphids suck the plant’s sugar-filled juices, causing a decline in vigor and stored reserves. A secondary problem associated with heavy aphid infestation is black sooty mold. This mold is a fungus that grows on the sweet honeydew that aphids leave on the leaf surface. The mold can reduce the photosynthetic capacity of the leaf. Also, it is unpleasant to work in orchards with sticky, honeydew-covered leaves.
There are two aphid species that attack hazelnuts in Oregon: filbert aphids (Myzocallis coryli) and hazelnut aphids (Corylobium avellanae). The two species can be visually distinguished by the following characteristics:
• Filbert aphids have smaller cornicles (small appendages on the back rear side of the aphid) that are often not visible with the naked eye. The antennae and legs are the same color as the rest of the body.
• Hazelnut aphids are often difficult to see because they are very similar in color to hazelnut leaves (Figure 4). The cornicles are more visible, and the antennae and legs are darker than the body. Hazelnut aphids can often be found feeding on the husk (Figure 5).
Filbert aphids were first reported in the United States in 1903 and probably arrived from Europe on filbert seedlings in the late 1800s. Hazelnut aphids were first documented in Oregon hazelnut orchards in 2003.
Filbert aphids overwinter as eggs on the tree trunk or branches (Figure 6). Eggs usually begin hatching around the beginning of March, and egg hatch continues for 3–4 weeks. Young aphids molt four times and then become adults that give birth to young aphids without sexual reproduction. As many as 10 generations of aphids are produced during the summer. Populations seem to decline naturally in the heat of the summer. In the fall, winged forms are produced, and these lay eggs.
Researchers have set different action thresholds for aphids by month (Table 1). Include both aphid species in your field scouting counts. If there are nut clusters on the limb that you choose to inspect for aphids, count the aphids on the cluster as well as those on the leaves.
In the early 1980s, Oregon State University researchers imported the aphid parasitoid Trioxys pallidus (Figure 7) from Europe, reared it, and released it in Willamette Valley hazelnut orchards. This parasitoid can spread as far as 10 miles from established populations. Today, nearly every hazelnut orchard shows signs of the parasitoid’s presence, usually accompanied by a reduction in the number of filbert aphids (Figure 8).
Leafrollers
Two leafroller species occur in hazelnut orchards in the United States: filbert leafroller (Archips rosanus Linnaeus) and obliquebanded leafroller (Choristoneura rosaceana). Filbert leafroller is native to Europe and was introduced accidentally to the United States in 1915. Obliquebanded leafroller is native to the United States.
Filbert leafrollers overwinter as eggs and hatch in late March or early April. Newly hatched larvae feed on both sides of new leaves. They soon pull the leaf into a roll around themselves and continue to feed for 4–8 weeks. They pupate in the webbed leaf, usually in late May or early June in the Willamette Valley (Figure 9).
Filbert leafroller adults (Figure 10) emerge from the pupae from late June or early July through mid-August. Adults live only 2–4 weeks. Before they die, they lay eggs in masses on trunks and major branches, where the eggs overwinter. On average, there are about 50 eggs per egg mass. Only one generation of filbert leafroller is produced each year.
Obliquebanded leafrollers overwinter as inactive and partially grown larva in the crevices and cracks of tree bark and on ground debris. As the temperature warms in the spring, overwintering larvae start to become active. In March and April, they feed on buds and new leaves. During late April or May, they pupate in the leaves. Pupation lasts 4–6 weeks.
Obliquebanded leafrollers adults (Figure 11)emerge from the pupae in early June through mid-July, when emergence usually peaks sharply. Adults mate and lay eggs within a few days of emergence. Eggs hatch within 2 weeks. The new larvae are often found in orchards by July 1 and feed for 6–8 weeks. In addition to eating leaves, larvae can cause nut damage by feeding on the shell underneath the husk, causing scarring and staining of young nuts. After their feeding stage, larvae pupate inside silk webs.
Adults of the second generation emerge 1–2 weeks after pupation. This emergence usually occurs from the first week in September through early October. The adults mate and lay eggs that hatch in 7–10 days. The larvae feed briefly and then overwinter until the following spring.
Action thresholds are the same for both leafroller species (Table 1). For obliquebanded leafrollers, check the nut cluster in July and August for second-generation larvae. Spraying is recommended if you find larvae feeding on the nut cluster and traps have high adult counts.
Winter moth
Winter moth (Operophtera brumata; Figure 12) is an occasional pest in hazelnut orchards. It emerges in the early spring and feeds on new leaves, giving them a characteristic tattered look.
Omnivorous leaftier
Omnivorous leaftier (Cnephasia longana; Figure 13) is a pest only on newly planted trees. It feeds on developing buds, damaging leaves and shoots.
Filbert bud mites
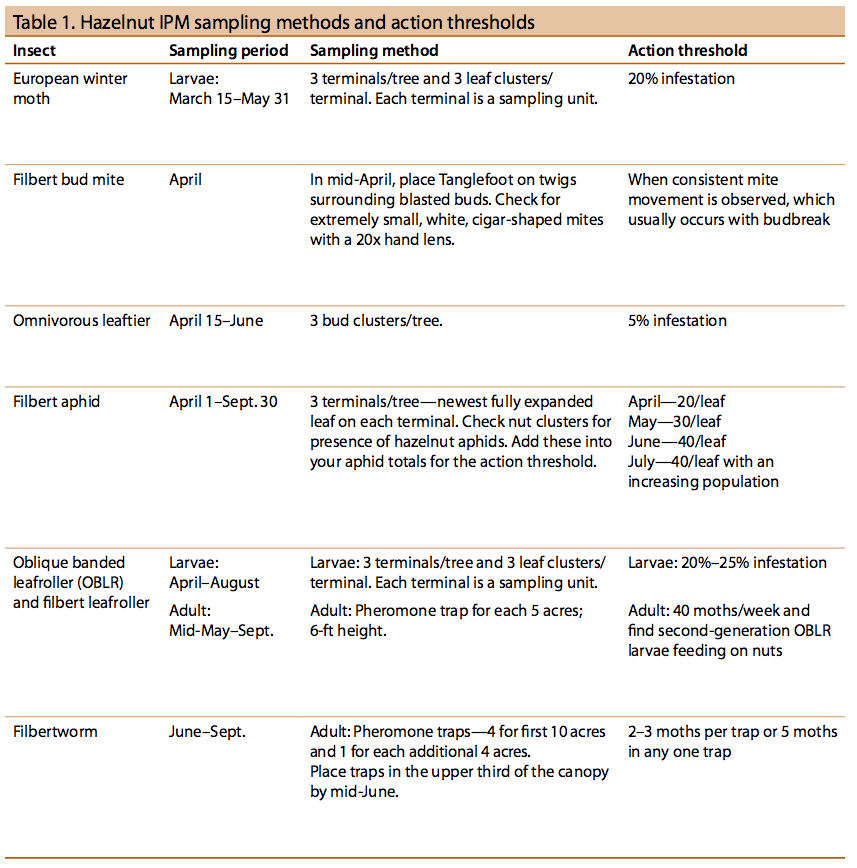
There are two filbert bud mite species in hazelnut orchards: Phytocoptella avellanae Nal. and Cecidophyopsis vermiformis Nal. Filbert bud mites are nearly translucent, cigar-shaped, microscopic organisms. They cannot be seen with the naked eye but can be detected by the damage they cause.
The mites damage female flowers as well as leaf buds. Hazelnut varieties differ in susceptibility to bud mite infestation. Damaged buds swell into “big buds”, also called blasted buds, as a result of mite feeding inside (Figures 14 and 15). The blasted buds dry up and eventually fall. Infested flowers do not produce fruit. Catkins become distorted, rigid, and brittle and produce little or no pollen.
Observations indicate that these mites are distributed throughout the nut-growing areas of the Pacific Northwest and commonly found on wild hazel. Both bud mite species cause blasted buds, but at different times (Figure 16).
Some growers and scouts have had trouble monitoring twigs for the presence of mites (Table 1). From a practical standpoint, most growers with varieties susceptible to bud mite watch for the buildup of blasted buds. When they feel that populations are significant, they use a control spray. On varieties that get sprayed for eastern filbert blight, growers can tank-mix a miticide with the fungicide spray. Caution: Always read and follow label directions when tank-mixing materials.
Brown marmorated stink bug
Brown marmorated stink bug (Halyomorpha halys; Figure 17) is a highly destructive invasive pest of annual and perennial crops in the eastern United States and an increasing threat in the Pacific Northwest. This generalist pest is also found in urban areas interspersed among high-value specialty crops, including hazelnuts. Brown marmorated stink bug was found in Willamette Valley hazelnut orchards during 2012 but is not currently known to cause economic injury. It can feed on reproductive plant structures, such as fruits and nuts, causing blank nuts as well as corking (Figures 18 and 19). It may also feed on leaves and stems.
Disease management
Several fungal and bacterial pathogens attack hazelnuts, but only a few are significant enough to require regular management. Eastern filbert blight and bacterial blight are common diseases that all growers should pay attention to. Diseases such as root rots or wood decay are not as common but can be troublesome in specific sites or situations. Many leaf spots and witches’ brooms are curiosities and do not need specific management. This section describes disease problems that are most important for growers to manage.
Eastern filbert blight
Eastern filbert blight (Anisogramma anomala) is a fungal disease. Historically, the pathogen had been restricted to eastern North America. In 1970, eastern filbert blight was found in a hazelnut orchard near Vancouver, Washington, and has since spread southward through the Willamette Valley and northward into British Columbia. Most hazelnut trees—including commercial orchards, backyard trees, and escaped seedlings—are at risk of contracting this disease.
Symptoms and signs
Eastern filbert blight is difficult to find by casual inspection. Usually, the fungus has been in an orchard for 4–5 years before it is first found. Symptoms can occur on any portion of the tree from the top of the canopy to the main scaffold limbs (Figure 20). The first cankers, however, are usually located on small branches near the top of the canopy.
Infected branches may suddenly die from July to September. Dead leaves may remain attached to the branch. Elongated, raised bumps begin to form on infected twigs and branches during June. When the bark is removed, the cambium below these bumps is chocolate brown.
These bumps continue to expand until the fungus breaks through the outer bark in July and August. A white, oval- to football-shaped fungal structure called a stroma can then be seen. As the stroma continues to mature from August to October, it turns black and is raised about 1/8 inch above the branch (Figure 21). Stromata occur in relatively straight rows lengthwise along the branch. Cankers can occur on branches of any size.
The disease may be confused with Eutypella cerviculata, which produces smaller, black fruiting bodies on dead branches. This Eutypella fungus produces black rings under the bark, which can be detected by using a pocketknife. Cicada egg-laying scars can also look somewhat like eastern filbert blight, but they are not black and look “stitched.”
Disease cycle
The eastern filbert blight disease cycle (Figure 22) requires 2 or more years, including a 12- to 15-month latent period when no visible symptoms can be detected. In the spring, spores are forcibly ejected and released in a sticky, white ooze during wet weather. Wind-driven rain and splashing droplets spread the spores to young, developing shoots. Infection occurs during wet weather from budbreak through shoot elongation. Spores penetrate immature epidermal cells just below the meristem, where cell elongation occurs. Neither wounds nor natural openings on hazelnut trees have been shown to be sites of entry for this fungus. The fungus is not spread via the nuts.
Stromata first begin to develop during the second summer after infection. Embedded within each black stroma are 50–100 flask-shaped perithecia. About 5,000 asci (sacks), each with eight spores (ascospores), are produced within each perithecium. The ascospores begin to mature in the fall as the rainy season begins. Several hours of continuous rain are needed for release of ascospores. Stromata will continue to sporulate, even after the diseased branch has been removed from the tree, until the canker has dried out completely. Ascospores are shed all winter long but cannot infect hazelnuts until the spring. Cankers on large scaffold limbs, trunks, or some resistant varieties may not produce stromata.
New stromata develop each year as the canker continues to expand around and along the branch. Cankers enlarge along the branch each year anywhere from a few inches on small branches up to 3 feet on larger branches of susceptible trees. Branch dieback occurs when expanding cankers girdle branches and limbs. Numerous new infections also occur each succeeding spring.
Most of the canopy dies on susceptible trees within 7–15 years after the first infection. Suckers, however, may be produced for many years. Vigor and health of an infected orchard decline slowly at first. Tree productivity also declines slowly at first but then sharply after 3–10 years, depending on the variety. At some point, the orchard becomes economically unproductive because the more susceptible pollinizers or main varieties die, resulting in poor nut set.
Variety susceptibility
The pollinizer variety Daviana and varieties Ennis and DuChilly are highly susceptible to eastern filbert blight. Most wild or escaped seedlings are also susceptible because they may have Daviana as a parent. The variety Casina is susceptible, as is Negret. Main industry varieties Barcelona, Butler, Hall’s Giant, and Willamette have intermediate susceptibility. Varieties Lewis and Clark have quantitative resistance but can become infected through repeated exposure. Contorted ornamental hazelnuts can also be infected. The native Corylus cornuta var. californica and C. cornuta var. cornuta and the Turkish hazel (C. colurna) do not appear to be susceptible.
An integrated approach using several cultural and chemical techniques is needed to adequately control this disease. The most effective management tactic is to plant new orchards with resistant varieties. Several are now available that have the single dominant Gasaway resistance gene, including Wepster, Dorris, Jefferson, and Yamhill. These varieties may get cankers, but the cankers may be fewer or smaller, may not produce spores, and may heal over in succeeding years.
For existing orchards, replace susceptible pollinizers with highly resistant pollinizers such as Gamma, York (compatible with Barcelona), and Felix—all of which have the single dominant Gasaway resistance gene. Also, reduce the number of susceptible pollinizers to 4% or 5% of the trees in the orchard. Remove or destroy escaped seedlings and trees beyond the perimeter of the orchard.
Scouting for cankers twice a year is very useful since early detection will aid overall control efforts. When pruning out cankers, remove infected branches 1–3 feet below the cankered area, and burn or bury these branches before budbreak in the spring. Completely remove severely infected trees. Also, start sucker control early in the season.
Thoroughly inspect new orchards for 2 years after planting to find trees that may have been infected before planting.
A total of four fungicide applications are recommended to adequately protect susceptible trees. Make applications starting at bud swell to budbreak and continue at 2-week intervals until early May. Thorough coverage of all branches is essential. Each row should be sprayed; however, alternate-row applications may provide acceptable coverage only for the first application. The disease kills trees slowly, so you won’t realize the yield benefits from fungicidal protection for 3–4 years after application. Alternate or tank-mix materials from different mode-of-action groups. Addition of a surfactant, if allowed by the label, will improve disease control.
Bacterial blight
Hazelnut bacterial blight (Xanthomonas arboricola pv. corylina) is the second-most significant hazelnut disease in the Pacific Northwest. Its prevalence and destructiveness vary from year to year. The disease usually causes the worst problems in years following heavy fall rains.
The most serious phase of bacterial blight causes trunk girdling (damage to the cambium layer of the bark) and death of trees up to 5 years of age (Figure 23). Trunks of stressed trees as old as 12 years are sometimes infected. Buds and nut-bearing twigs in the tops of trees often are killed, thereby reducing nut yield. The disease is seldom found on the nuts or in the roots.
You can reduce bud and twig infections significantly in both young and old orchards by using copper-based products in the fall. Apply sprays in late August or early September before the first heavy rains. If fall rains are heavy, apply another spray when 75% of the leaves have dropped. Note that most copper-based products do not allow use until after harvest. A compatible spreader-sticker improves the coverage and adherence of copper-based materials, thus increasing their effectiveness.
Trees that are stressed in the first year after planting are much more likely to become infected and die. Reduce stress by planting pathogen-free nursery stock in early winter. Do not let roots dry out. Apply mulch around the base of newly planted trees with chipped, composted tree debris to reduce moisture stress. Control sunscald during summer using a shield or white paint on trunks. Irrigation in the first year will also result in lower tree mortality from this disease.
Prune out infected twigs and branches when you find them. Make cuts 2–3 feet below affected branches. Clean pruning tools between cuts with a registered disinfectant.
Root rots
Hazelnuts have a variety of poorly investigated root problems. Some, such as Armillaria root rot and Phytophthora root rot, are due to specific pathogens. Others, such as wet feet, are very general and may not specifically involve pathogens.
Armillaria
Armillaria mellea, a fungus, can infect tree roots, killing the cambium and decaying the underlying xylem. Often found on newly cleared land, this root pathogen is native to the Pacific Northwest, where it occurs on the roots of many forest tree species, including Douglas-fir, madrone, oak, willow, and yellow pine. The host range includes more than 500 species of woody plants, making its common name of “oak root fungus” slightly misleading.
The fungus spreads vegetatively below ground, which leads to the formation of disease centers (groups of dead and dying plants). The fungus can survive on woody host roots long after the host dies. Its mycelium (vegetative fungal tissue) decomposes root wood for nutrients as it grows. When infected plants are removed, infected roots that remain below ground serve as a source of inoculum for trees planted in the same location.
Infection occurs when hazelnut roots come in direct contact with partially decayed tree roots and are colonized by mycelium. Infection can also occur when tree roots contact rhizomorphs (black, shoestring-like fungal structures) that grow out from partially decayed roots and through the soil. Once tree roots are infected, whether they are living or dead, they serve as a source of inoculum for neighboring trees.
The first indication of infection is usually poor shoot growth together with premature leaf drop (Figure 24). One part, side, or section of the tree may be affected at first. This corresponds to the side that is supported by the roots first attacked by the fungus. Trees may live for several years before finally dying. White mycelial plaques or fans can be seen on the main roots or root crown when the outer bark is removed (Figure 25). Rhizomorphs may also be found clinging to the bark in this area. These appear as dark brown to black, branching, rootlike structures.
When clearing a new site of forest trees and shrubs or when clearing a site with infection centers (infected plants), take the following precautions:
• Girdle large trees before removal to hasten decay of roots.
• After removing aboveground vegetation, clear soil of stumps and large roots.
• Deep-rip the soil in more than one direction to bring large roots to the soil surface.
• If possible, remove all roots larger than 1 inch in diameter from the soil.
• Burn all woody debris.
• Leave this ground fallow at least 1 year.
Permanently removing soil in a 3-foot radius around the crown and main trunk root area has been effective in citrus and other tree fruits grown in California and Australia and may be of benefit for managing infected trees in the Pacific Northwest. Also excavate root collars of healthy looking trees surrounding disease centers. Be sure to keep root collars free of soil. Remove and destroy severely infected trees, being careful to remove as much root material as possible from soil. If practical, do not replant where infected trees have been removed.
Phytophthora
The Oregon State University Plant Clinic has detected the fungus-like microorganism Phytophthora in the roots of dying trees. Although this is considered rare in hazelnuts, Phytophthora is a common root rot organism found on many other plants growing in saturated soil conditions.
Phytophthora infection can result in discolored roots and vascular cambium as it colonizes the roots and root crown (Figure 26). Mature trees may develop a dark, wet stain on the lower trunk. These areas may weep a dark fluid in the spring. Diseased and discolored cambium will extend down to and below the ground rather than up and into the branches. General decline symptoms of yellowing, branch dieback, and nutritional deficiency will occur as a result of the lack of functioning roots. Trees may show lack of vigor and suddenly crash during hot summer weather.
There has been little research on this root rot in hazelnuts, but there are some tactics to avoid and manage this disease. Plant trees on well-drained soils. Use drain tile in low areas of the orchard to improve water drainage. Avoid reusing pots from a previous crop for propagation. If you must reuse pots, wash off all debris and soak pots in a sanitizing solution or treat them with aerated steam for 30 minutes. Products that are effective on other crops might control Phytophthora root rot on hazelnuts, but use these products only when you’ve confirmed the diagnosis. Chemicals will not keep heavily infected trees from dying.
Wet feet
Hazelnuts will not tolerate saturated soil conditions for extended periods of time during the winter. Trees grow poorly under these wet conditions and may die. Trees growing in low areas of the orchard are of low vigor and grow more slowly than other trees in the orchard. Trees may survive for many years in these areas, but they remain stunted and do not produce as many nuts. Some trees will continue to decline, with twig and branch dieback and, eventually, death. Cultural control tactics used for Phytophthora root rot will also help these trees.
Wood decay
Wood decay leads to limb breakage, uprooted trees, trees that are broken at the soil line during windstorms, or decreased tree vigor and dieback.
Most fungi that cause extensive wood decay of nut trees produce spore-bearing structures, such as mushrooms and conks (Figure 27). Spores are forcibly discharged and usually disseminated by air currents. Wood-decay fungi enter trees through wounds that expose sapwood or heartwood. Injuries from pruning, sunburn, lightning, or cultivation equipment can expose susceptible wood. Large wounds as well as stub and horizontal cuts are often entry sites for these decay fungi. When spores land on susceptible wood, the fungus colonizes and uses the wood as a food source. New mushrooms or conks develop after extensive colonization of the wood and under suitable environmental conditions.
Wood decay is generally observed in older trees after extensive fungal colonization, but it can also occur in young trees. Decay fungi frequently function as secondary invaders after a succession of microorganisms, including bacteria, yeasts, and other fungi. Decay in a single tree may be caused by more than one fungal species. Two types of wood decay occur in living trees:
• White rots cause moist, soft, or spongy wood that is a lighter color than sound wood. All major structural components of the cell wall—cellulose, hemicellulose, and lignin—are degraded. The strength of white rotted wood decreases only in advanced stages of decay.
• Brown rots are brown, dry, and crumbly, with longitudinal and transverse cracks. Most of the cell wall polysaccharides are degraded, but the lignin component is unchanged or slightly modified. The strength of brown rotted wood is significantly reduced or lost entirely shortly after decay begins. These rots are usually in exposed wood and can cause extensive damage resulting in structural failure.
Help prevent wood decay by using cultural practices that promote tree growth and vigor while minimizing injuries that expose wood. Irrigation water, especially from sprinklers, should not wet the trunks. Cultivation and mowing equipment should not injure the roots, crown, or lower trunk. Make pruning cuts adjacent to, but not into, the supporting branch, and prune when branches are small to enhance callus formation and wound healing.
Lichen and mosses
Lichens are a fungus and an alga living in association with one another to give the appearance of a single plant. They occur in several forms, such as crusty gray, green, yellow, and white growths. Some are leaflike; others resemble a tuft of horse hair hanging from the branches. Mosses are green plants that are somewhat similar to algae, except they have a complex structure that resembles stems and leaves. All these plants contain chlorophyll and make their own food, so they do not directly injure the plants on which they grow.
Although lichens and mosses do not feed on trees, they collect moisture that increases the weight of the limb during freezing weather. This causes limb breakage during heavy, wet snows or ice storms.
If you regularly prune or spray the orchard, lichens and mosses are generally not a problem. If you do not need to spray plantings regularly for other reasons, spray thoroughly every 4–5 years to control lichens and mosses. Spray when lichens and mosses are actively growing. Application of copper-based materials after harvest in the fall may also provide some bacterial blight control. Dead lichens and mosses will stay on trees for a time but gradually weather away.
Kernel defects
There are several defects that affect the quality of kernels and in-shell hazelnuts.
Blanks
The official definition of a blank is a shell containing no kernel at all or a kernel that fills less than one-fourth the capacity of the shell. Pollination stimulates the shell to develop, but the kernel, for unknown reasons, fails to develop normally. The kernel either fails to grow at all or starts to grow and then aborts.
Lack of pollination is not the cause. If pollination had failed, the shell would not have developed at all. The variety Barcelona produces a higher proportion of blank nuts than other varieties. Blanks generally fall from the tree earlier than shells with kernels. Shells are much easier to crack open and may become necrotic. Flail the orchard floor as the first few nuts drop from the tree to reduce the number of blanks found at harvest.
Brown stain
The cause of brown stain is unknown. Since no insects or pathogens have been associated with this disease, it may be due to a physiologic problem. The severity of the problem changes from year to year. Brown stain is characterized by a brownish liquid that soaks the side or end of the nut. Staining begins when nuts are about half grown. The first symptoms are water-soaked spots between the vascular bundles on the shell that are softer than normal (Figure 28). Soon spots become brown to dark brown and sunken. Internal areas are affected later, producing a water soaking around the developing kernel. The entire interior of the shell, including kernel, may turn into a soft, brown, watery mass. Kernels may also be misshapen.
Mold
Mold is defined as any visible growth of mold on the outside or inside of the kernel. In practice, any white, fuzzy, mycelial growth is classified as mold. Many of the following fungal problems would only be classified as decay or discoloration in the absence of active mycelial growth.
Several fungal species are consistently associated with kernel molds. Ramularia (asexual stage) of Mycosphaerella punctiformis is consistently isolated from kernels with tip mold. A Phomopsis species and Septoria ostryae (formerly S. coryli) are commonly associated with internal discoloration. Nematospora coryli, a yeast, is consistently associated with the disease known as kernel spot. Several other fungi have been isolated from shriveled hazelnuts. These organisms may be secondary opportunists that invade kernels that have been stressed physiologically, but all have been associated with kernels at shelling.
In Oregon, moldy kernel incidence averages 0.5%–1% annually. Mold incidence in individual orchards is frequently much higher (3%–10%). Molds reduce kernel quality, but the degree varies with the causal agent and environmental conditions during symptom development. Mold is often highest if rains are significant in spring or during harvest. Mold can also be a problem when bins filled with nuts are grouped together and left out in the rain before drying. Varieties Lewis and Santiam generally have higher levels of mold than Barcelona in any particular year.
Lesions on the kernel surface characterize kernel spot. Lesions are dark, sunken, and varied in size and shape. All surfaces of the kernel can be affected. Kernel shriveling in combination with sporulation of fungi on the kernel surface is also a symptom.
To reduce the incidence of mold problems, delay mechanical flailing of the orchard floor until as late in spring as practical. Harvest, dry, and shell kernels quickly after they fall to the ground. Harvesting before fall rains has been associated with reduced mold counts. Keep totes or boxes full of harvested nuts out of the rain.
Miscellaneous defects
Chafing or scraping. Kernels are normally covered with a brown skin. During shelling or sorting, kernels may come into contact with each other or with the machinery with enough force to remove the skin and affect the kernel. This is scored as damage when it extends into the meat and affects more than one-eighth of the surface area, and as serious damage when it exceeds one-fourth of the surface area.
Discoloration. Black discoloration of the external kernel surface or internal cavity that materially affects the appearance of the nut is considered discoloration. Light or dark brown colors are not considered or scored in this category.
Rancidity. Kernels are scored as rancid only if they taste noticeably rancid. A stale flavor or an oily or creamy kernel appearance does not necessarily indicate rancidity.
Shrivel. Kernels may shrivel as a result of moisture loss. This defect is scored as damage when the kernel is materially shrunken, wrinkled, and tough. The kernel is considered seriously damaged when it is seriously affected by shriveling.
Decay. Kernels or portions of kernels showing decomposed areas are scored as decay.
Vertebrate pests
Blue jays and squirrels
Blue jays (stellar and scrub jays) and squirrels often congregate in large numbers around hazelnut orchards. Ground squirrels store nuts in their burrows and consume large quantities of the crop. Up to 40 lb of nuts per burrow have been observed in hazelnut orchards. There are also tree squirrels in hazelnut orchards. They often do damage high in the tree by girdling branches. Trapping and baiting are often used to manage vertebrate pests.
Shooting is also a common method of controlling these animals, but it is costly and time consuming. Trapping jays and squirrels with ordinary steel traps made for rodents has been fairly successful. Place traps for ground squirrels over the burrows. Traps for tree squirrels and blue jays are most effective when attached to the top of a post. Use 2-foot-high posts for tree squirrels and 8-foot-high posts for blue jays. Prop the posts against trees along the outer row of the orchard. Place a hazelnut in the trap; nuts are the only successful bait for stellar and scrub jays and squirrels.
Deer
Deer are particularly serious pests of young hazelnut trees in orchards near wooded areas. So far, there is no satisfactory method of preventing deer damage. The most reliable solution, although expensive, is to install deer fencing around the entire orchard. Hunting deer is another way to reduce damage to hazelnut trees. In some locations, you can obtain special predation permit hunting licenses for this purpose.
Repellents have been partially successful in deterring deer. Bags of blood meal, bone meal, or soap hung on trees are the most common. When replaced every 2 or 3 months, they are somewhat successful. Several chemical repellent sprays have been successful in some instances. As the trees grow, repeat sprays are needed to protect new foliage
Gophers and moles
A single gopher can kill a dozen or more trees, usually young ones, in one season by chewing on roots or girdling the bark around the trunk. Crescent-shaped mounds of earth with either an open or plugged hole might indicate gopher activity.
Gophers are usually attracted to the orchard by the succulent roots of a cover crop. When the cover crop is turned under, gophers turn their attention to the trees. There are three methods of controlling pocket gophers:
• Baiting. Over large or heavily infested areas, baiting is the fastest and most economical method of control. The best time to bait gophers is in early spring, before the young are born. Bait only where there is fresh gopher activity. Stragglers that are not poisoned will continue to produce fresh mounds. These gophers can be trapped.
• Burning sulfur in the hole. Remove the plug of soil that covers the hole of a fresh gopher mound and place several tablespoons of sulfur in the bottom of the hole. Put the sulfur in the hole before the gopher plugs it. Then light a propane weed burner and aim it at the sulfur, burning the sulfur and blowing the flames down the hole. Be careful when working around the burner.
• Trapping. Unlike gophers, moles don’t damage trees, but they make mounds that interfere with mechanical harvesting. Mole mounds are circular (gopher mounds are crescent-shaped) with a hole in the center. Trapping is the most effective way to kill moles.
Meadow mice (Voles)
Meadow mice (voles) leave holes in the orchard floor (Figure 29). Meadow mice usually damage hazelnut trees by chewing on and girdling the roots and stems. They depend on cover for protection, and damage usually occurs when there is heavy sod, a cover crop, litter, or snow near the trees.
Poison baits are the most effective, economical, and least hazardous method of controlling meadow mice. Grain coated with a registered pesticide is the most widely used vole bait in the Pacific Northwest.
Bait location is important. Meadow mice stay near their established runways, so place the bait directly in burrow entrances or runways. Runways are visible as well-worn, narrow pathways through orchard floor vegetation. Burrow entrances are marked by well-defined, open holes in the ground (no mound). You can make a false cover by placing a piece of cardboard or tar paper over the burrow entrance or in the runway. This cover provides a protected dining area. To prevent accidental poisoning, keep pets and children out of an orchard that has been baited.
For more information
Many Oregon State University Extension publications on hazelnut production and pest management are available through the OSU Extension Catalog
This information is provided for educational purposes only. If you need legal [or tax] advice, please consult a qualified legal [or tax] adviser.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.