Key points of this publication
- Presence of grapevine red blotch virus (GRBV) is confirmed in all winegrape-producing regions of Oregon.
- Spread of GRBV has been confirmed both spatially and temporally.
- GRBV incidence increased from two to 10 times annually in the Willamette Valley and Southern Oregon vineyards sampled and studied from 2014 to 2016.
- GRBV is difficult to control because it is not yet clear how the virus spreads.
- Despite anecdotal evidence and observation of various hopper insect species in infected vineyards, research has not proven that leafhoppers spread the virus. There is limited evidence of spread by treehoppers.
Introduction
Grapevine red blotch virus (GRBV) is one of about 80 viruses infecting wine grapes worldwide. It may affect fruit ripening and thus impact the quality of finished wines.
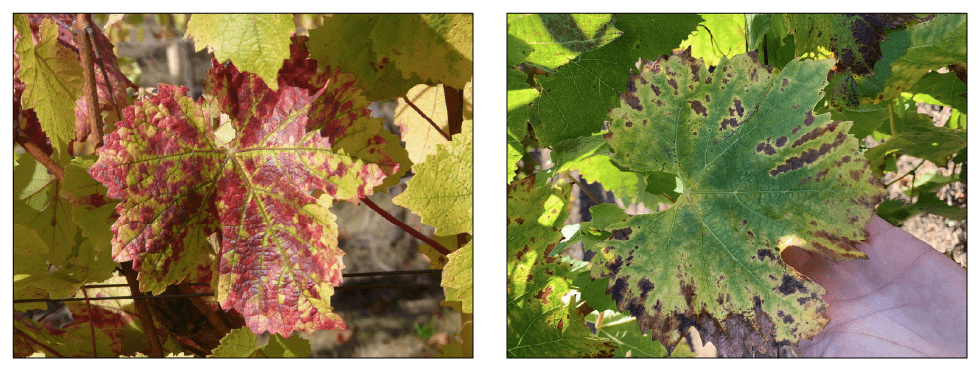
Beginning in 2012, Oregon vineyard managers were puzzled by vines testing negative for grapevine leafroll-associated virus (GLRaVs) yet displaying leafroll-like symptoms. The puzzle was solved in 2014 by the identification of GRBV in Oregon vineyards. Both GRBV and GLRaV cause similar red discoloration of leaves in red-berried cultivars. The distribution of the discoloration can differ between the two diseases, however. One main difference in disease symptoms is that GLRaV-infected grapevines tend to have curled leaves, while GRBV-infected grapevines remain flat.
Our study examined vine tissue samples collected in late summer or early fall in Southern Oregon, the Willamette Valley and the Columbia Basin wine-producing regions of Oregon. At the time of the study (2013–2016), no samples collected from a vineyard block in the Columbia Basin tested positive for GRBV, although a more recent survey by the Oregon Department of Agriculture confirmed the presence of GRBV in all regions of the state.
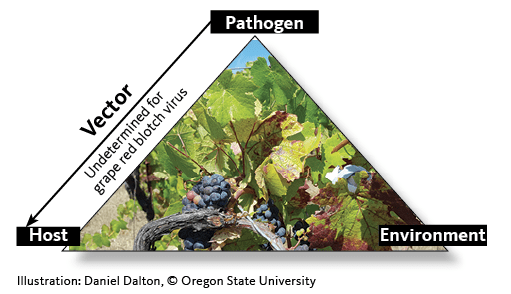
The disease triangle
The disease triangle is a classic model explaining the spread of infectious disease. The three components of the triangle are:
- The pathogen — the infectious organism.
- The host — the organism that becomes diseased.
- The environment — the physical conditions where the disease can persist (Figure 1).
A fourth component is present in the case of insect-transmitted diseases:
- The vector — the organism that delivers the agent to the host.
In the case of GRBV:
- The pathogen is grapevine red blotch virus.
- The host is the grapevine.
- The environment is the vineyard agroecosystem.
- The vector has not determined.
GRBV is a newly identified member of the geminivirus family of viruses. Geminiviruses are single-stranded DNA viruses that infect many crops of economic importance, including vegetables and cotton. To date, known geminivirus vector insects include whiteflies, leafhoppers and treehoppers.
Many grapevine species, cultivars and rootstocks are documented hosts of GRBV. The symptoms differ between red- and white-fruited grapes (Figure 2). You can find resources distinguishing symptom expression between red- and white-fruited cultivars at the end of this publication.
Expression of viral symptoms can be confounded by:
- Concurrent infection with GLRaV or other viral disease of grapevine.
- Nutrient deficiencies in the vineyard, including phosphorus, potassium or various micronutrients, may also result in red leaves.
- Girdling by tie tape or other mechanical damage.
- Specific combinations of cultivars and rootstocks.
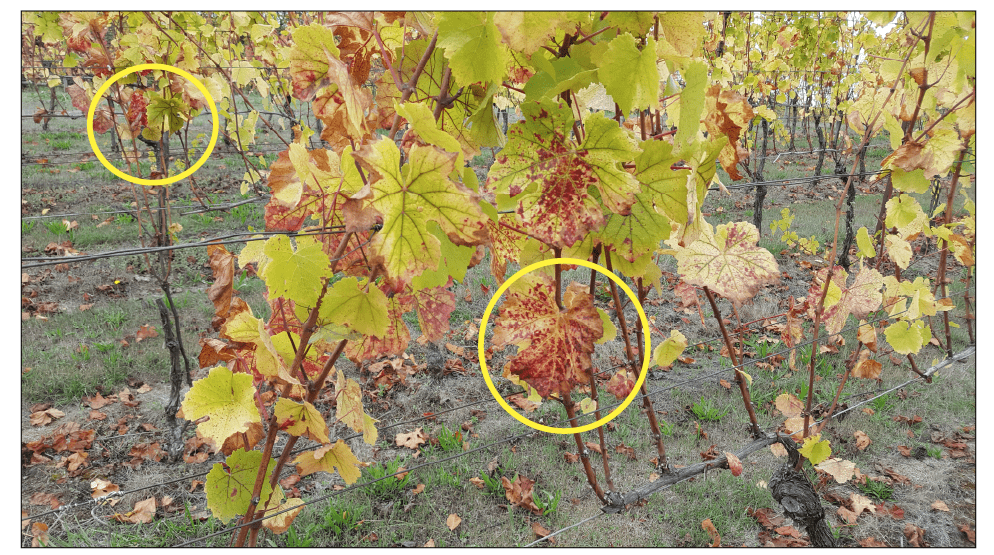
The identity of the vector spreading GRBV throughout vineyards remains a mystery. It is well-documented that infected rootstocks or budwood will introduce GRBV into the vineyard by way of the newly planted vines that are already infected. It is possible that the virus will spread to healthy vines if there is a vector. Through testing plant materials with polymerase chain reaction (PCR) (Figure 3), we have observed the virus spreading from the edges of vineyard blocks toward the interior sections (Figure 4).
Pruning shears are not known to transmit the virus from plant to plant. We do not observe obvious streaks of virus-infected vines in the rows, as we would if pruning shears spread the virus.
Multiple insect vectors have been assumed to spread the virus, including the three-cornered alfalfa hopper (Spissistilus festinus). Although grape mealybug (Pseudococcus maritimus) spreads GLRaV, field research has not supported that mealybugs can spread GRBV. Leafhoppers and treehoppers spread other geminiviruses. However, researchers have not been able to induce GRBV infection in the vineyard with leafhoppers, and only on occasion with great effort in the greenhouse with treehoppers. Thus, the role of leafhoppers, treehoppers or other insects as potential vectors of GRBV is unclear.
Testing for GRBV
Vineyard managers cannot rely on visual inspection of individual grapevines to differentiate GRBV infection from GLRaV infection. Virus diagnosis can only be confirmed by submitting tissue samples to a laboratory specializing in viral plant diseases. Tissue analysis is also a sure way to diagnose plant nutrient deficiencies. Whether the problem is virus- or nutrient-based, symptoms can be subtle and easily confused. Multiple disease or management problems can occur together or be mistaken for one another. Autumn is the best time of year to collect tissue samples for diagnosis of viral disease.
See the “Other resources” section of this document to find laboratories conducting tissue analysis diagnosis of these viruses. Always check with the lab before submitting samples to confirm how tissue samples should be collected and submitted.
These are terms used to discuss diagnosis of viruses in tissue samples:
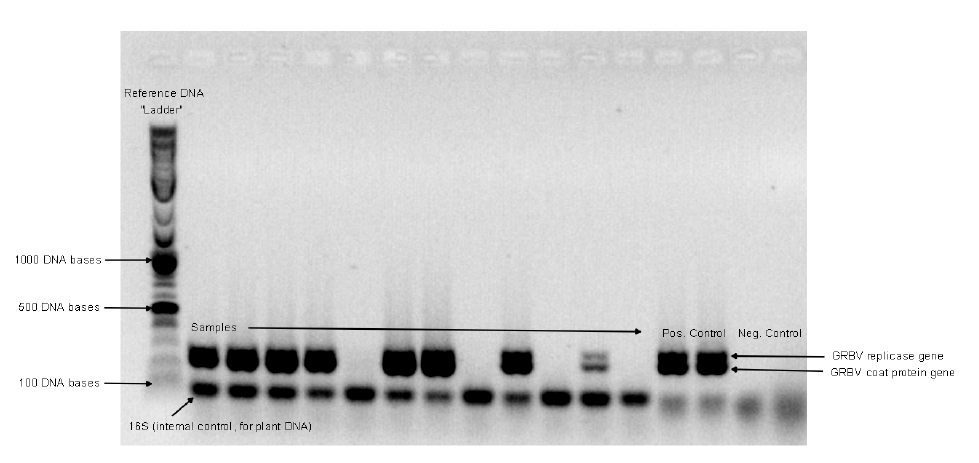
- Polymerase chain reaction (PCR) is a technique that duplicates a short fragment of DNA millions of times so that further analysis can be done. GRBV is a single-stranded circular DNA virus.
- PCR gel electrophoresis sorts molecules (like DNA) according to their size and charge. As the molecules travel via electrical current running through a gel medium, they group with like molecules, and form a pattern in the gel. Those patterns are printed out and serve as a fingerprint when compared to patterns formed by known viruses (Figure 3).
In our study, vines in four Oregon vineyards were assessed for GRBV infection using PCR and PCR gel electrophoresis analyses. The results of those analyses (positive or negative) were tied to the vines’ coordinates in the vineyard. Results of spatial analysis indicate the degree of randomness or order of the aggregation of infected vines in a vineyard.
Our results showed that GRBV spreads from a diseased vine to neighboring plants.
The spread of this virus within a vineyard from year to year showed significant relationships between the coordinates of vines testing positive for GRBV in the current year to the coordinates of vines testing positive in previous years.
Other resources
Bettiga, Larry. 2015. “Assessing Grapevine Leafroll and Red Blotch Disease Impacts in Local Vineyards.” Agriculture and Natural Resources, University of California.
Clean Plant Center Northwest, Washington State University, Prosser, Washington.
Foundation Plant Services, University of California, Davis.
Grapevine Red Blotch Disease. Levin, A.D. and N. KC Achala. 2020. “Water Deficits Do Not Improve Fruit Quality in Grapevine Red Blotch Virus-Infected Grapevines (Vitis vinifera L.).” Frontiers in Plant Science.
National Clean Plant Network for Grapes.
Oregon Department of Agriculture. 2016. “What Are The Requirements For Shipping Grape Nursery Stock Into Oregon?” Acquiring Healthy Grape Plants.
Oregon Department of Agriculture. 2016. Grapevine Red Blotch Disease.
Skinkis, Patricia. 2019. Red Blotch Disease: Virus Testing Labs on the West Coast.
USDA Farm Service Program Disaster Assistance. 2018. Tree Assistance Program.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.
© 2020 Oregon State University.
Extension work is a cooperative program of Oregon State University, the U.S. Department of Agriculture, and Oregon counties. Oregon State University Extension Service offers educational programs, activities, and materials without discrimination on the basis of race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, familial/parental status, income derived from a public assistance program, political beliefs, genetic information, veteran’s status, reprisal or retaliation for prior civil rights activity. (Not all prohibited bases apply to all programs.) Oregon State University Extension Service is an AA/EOE/Veterans/Disabled.