Introduction
An increasing number of growers are producing quinoa in Oregon’s Willamette Valley. This novel crop has historically had a high market value and adds diversity to crop rotations.
Quinoa has been the subject of much interest in the last 10 years as a potential crop throughout many regions of the U.S. and Canada, However, widespread production is only slowly beginning.
In the Willamette Valley, adoption of quinoa in cropping systems has been hampered by production, processing and marketing difficulties. To help address those challenges, we tested 17 quinoa varieties and four planting dates to identify the most suitable options for our region. We also tested harvesting and processing methods.
Background
Recent trends in health foods and gluten-free products have helped quinoa become mainstream, both as a grain substitute and in processed products like energy bars and chocolate desserts. While quinoa is a traditional food in South America, the U.S. is by far the largest importer of quinoa worldwide (FAOSTAT 2020) for use as a rice or grain alternative and as an ingredient in processed products. Large-scale manufacturers of products containing quinoa currently import quinoa from Andean countries because top-producing countries have intensified production dramatically in the last decade to meet global demand (Jacobsen 2011).
U.S. consumers, meanwhile, have favored local, farm-grown quinoa with distinctive quality attributes such as color and taste. These unusual quinoa varieties are typically more profitable for domestic farms than mainstream varieties of quinoa. However, market prices have dropped an estimated 40% to 75% from the record high prices of 2010–2012 due to increased worldwide production (Livingstone 2018, S.K. 2016).
In addition to price competition from imported quinoa markets, seed processing is a challenge for domestic quinoa production. The small seed size and thick stems make separating seeds from crop residue difficult without modifying equipment. Quinoa also produces compounds, called saponins, that coat the seed. Saponins are bitter and may have evolved to discourage feeding by insects or birds. Seeds destined for human food need to be processed to have saponins removed through abrasion and/or washing prior to packaging or processing into food products. This process is not currently available on a large scale for most regions in the U.S., although a handful of private companies are developing capacity in the western U.S.
The largest obstacle to widespread quinoa production is a lack of consistently high seed yields. In many areas of the U.S., quinoa trials have not consistently produced seed due to insect feeding damage and heat intolerance (Hinojosa et al, 2018). For example, many varieties of quinoa will not produce seed if summer temperatures rise above 95°F during flowering and early seed set (Murphy and Matanguihan, 2015).
However, the cooler, wetter areas west of the Cascades have produced more stable yields. As a result, recent research in the Willamette Valley has sought to identify best practices for production in the region, including the variety and planting date evaluations described in this publication.
Quinoa crop management
Field preparation should allow for shallow planting of one-quarter inch in a fine seedbed; quinoa planted too deeply will result in poor emergence. Most commercial production favors row spacing between 12 and 18 inches with dense within-row spacing sometimes as close as 1 inch between plants! The tight in-row spacing forces a single main stem, limits secondary seed heads, and helps bring the crop to maturity faster (Risi and Galwey 1991). Quinoa planted at lower densities has more branching and multiple seed heads with a wider variability of seed maturation dates.
Nutrient recommendations are wide-ranging. Weed competition trials under greenhouse settings suggest that quinoa is strongly competitive, scavenging nitrogen adequately for growth when weed species are present at both high and low nitrogen fertility rates (Buckland 2016). Therefore, lower rates of nitrogen (between 50 and 100 lb N/acre), are likely to be sufficient, depending on soil reserves.
Quinoa varieties can take 100–120 days to reach full maturity, so planting early in the spring seems advantageous. However, there are two potential considerations. First, quinoa generally does not thrive with excessive soil moisture. Fields with standing water from spring rains should be planted later. Second, weed control will be more challenging with an early planting date. In favorable conditions, quinoa usually germinates and emerges within a week of planting. After the first true leaves emerge, the quinoa plant canopy growth slows for two or more weeks while the root system develops. This slow canopy growth allows early season weeds to gain an advantage. In Oregon, early spring is often too wet to allow for frequent field access for weed cultivation. All of these conditions can lead to the “perfect storm” of weeds.
In our research trials in 2018, a quinoa crop planted on March 31 required significantly more hand weeding (estimated at 166 hours per acre compared with 41 hours per acre with May and June planting dates) than the other planting dates. Weed pressure decreased greatly with each later planting date. This is likely due to the faster growth rate of quinoa with warmer temperatures, which allows quinoa to outcompete weeds and provides an opportunity to terminate the first flush of weeds prior to planting quinoa. Similar results were noted in 2019; however, primary weeding was accomplished with tractor-mounted cultivation equipment and therefore time to cultivate was not recorded.
As this discussion around mechanical weed control suggests, there currently are no selective herbicides labeled for use in quinoa production. Several are under review through the national IR-4 Specialty Crops Program of the U.S. Department of Agriculture, but for now mechanical cultivation and possibly hand-weeding is almost assured. Good seedbed preparation to decrease weed emergence is critical for crop establishment.
Quinoa seed is mature when it is hard. An easy way to test is by pressing with fingers. Although the seeds are mature, the plants may still be upright with considerable moisture in the stems and leaves.
This slow process of plant die-back, or senescence, makes harvesting before winter rains difficult. But harvesting before any fall rain is crucial because mature quinoa seed that remains in the field during rain events will sprout, ruining the crop (Picture 3).
As a result, most commercial operations are using common seed production techniques for crop dry-down in order to harvest quinoa before rain arrives. In western Oregon, growers swath the field to terminate the crop and hasten dry-down so that it can then be picked up and run through a combine easily. In late August, a swathed field can dry within a week. Swathing and drying in the field has drawbacks, however; the process results in seed shatter that may cause a significant loss of yield and a source of volunteers in the next crop. Work to develop approved desiccants needs to be a priority so growers can directly combine the crop. Smaller-scale growers have harvested by hand, cutting and drying on tarps, then threshing.
Seed cleaning
Both mechanical and hand-harvested seeds require additional steps after harvest to remove chaff and saponins. Raw seeds are inedible because of the astringency of saponins in the seed coat. Commercial seed-cleaning equipment can be modified for the small seed size and to include a cleaning step to remove saponins. Currently, the nearest large-scale processors for Oregon farms are in Idaho and California, requiring long-distance shipping at high costs. Recent work has begun to develop seed-processing capacity in the Willamette Valley. Smaller-scale producers — those mostly producing quinoa as a seed crop — have been successful with the first step of processing. That first step includes separating chaff by using fans or even by vacuuming seed heads with a clean high-speed industrial vacuum with an extra-long hose attachment. The vacuum pressure moves the seeds along the tube, which provides light abrasion as the seed travels the length of the tube. However, additional processing for saponin removal remains a problem for food crops and large-scale production.
Variety and planting date trial in the Willamette Valley
Because temperature during flowering is one of the biggest factors influencing yield, research in 2018 and 2019 compared the yield of several varieties across multiple planting dates. Planting dates were based on seasonal conditions. The first planting date immediately followed spring soil drying. Three additional planting dates were spaced out every three to four weeks for a total of four planting dates in each year.
The quinoa trials were conducted in a half-acre field at the OSU North Willamette Research and Extension Center in Aurora, Oregon. The plots included 10 quinoa varieties (Table 1) in 2018 and 17 varieties in 2019, planted on four different dates (Table 2). Each year the seed was purchased or obtained from four sources: Wild Garden Seed (Philomath, Oregon), Adaptive Seed (Sweet Home, Oregon), Washington State University (Pullman, Washington), and Oregon State University (Corvallis, Oregon). Some of the varieties used in the trials are not currently commercially available. Each variety was seeded at a rate of 7.9 lb/acre in plots that were 15 feet long and three rows wide with 18-inch spacing between rows. Each planting date had four replicates of each variety.
Table 1. Quinoa varieties 2018 and 2019
|
2018 |
2019 |
||
|
Varieties |
Seed provided by: |
Varieties |
Seed provided by: |
|
Oro de Valle |
OSU |
Oro de Valle |
OSU |
|
Kaslaea |
WSU |
Kaslaea |
WSU |
|
Titicaca |
WSU |
Titicaca |
WSU |
|
Puno |
WSU |
Puno |
WSU |
|
QQ74 |
WSU |
QQ74 |
WSU |
|
Cherry Vanilla |
Wild Garden Seed |
Cherry Vanilla |
Wild Garden Seed |
|
Ivory |
Wild Garden Seed |
Ivory |
Wild Garden Seed |
|
Mint Vanilla |
Wild Garden Seed |
Mint Vanilla |
Wild Garden Seed |
|
Red Head |
Wild Garden Seed |
Red Head |
Wild Garden Seed |
|
Bio Bio |
Wild Garden Seed |
Bio Bio |
Wild Garden Seed |
|
Kaslala Multicolor |
Wild Garden Seed |
||
|
Cocoa Cherry |
Wild Garden Seed |
||
|
Peppermint |
Wild Garden Seed |
||
|
Buffy |
Wild Garden Seed |
||
|
Chadmo |
Adaptive Seeds |
||
|
Dave 407 |
Adaptive Seeds |
||
|
Linares |
Adaptive Seeds |
||
Quinoa was planted monthly beginning mid-March each year. The second, third, and fourth planting dates were in mid-April, mid-May, and mid-June, respectively (Table 2). Plants were observed throughout both growing seasons to assess flowering and seed head development (Table 2). Plots were maintained by a combination of hand-weeding and mechanical cultivation, and were irrigated every 7–10 days through the soft-dough stage of seed development, generally at a rate near the reference evapotranspiration rate as monitored by AgriMet weather equipment (US.usbr.gov/pn/agrimet/agrimetmap/araoda.html). Previous studies show irrigation is effective in maintaining yields in critical crop development phases and in years with little rainfall (Geerts et al, 2008; Buckland et al, 2019).
Seed was harvested beginning in late July 2018 and mid-August 2019. Plants in each plot were harvested by hand and dried. In 2018, seed heads were stripped off of quinoa stalks and run through a small plot combine (Wintersteiger Nursery-master Elite 2000) to separate seeds from chaff. In 2019, seed were removed from chaff manually (described in seed cleaning section below).
Table 2. Planting dates and crop phenological development
|
2018 |
2019 |
||
|
Planting date |
Flowering period |
Planting date |
Flowering period |
|
Mar 31 |
Late June |
Mar 22 |
Mid-late June |
|
Apr 24 |
Mid-July |
Apr 18 |
Early July |
|
May 18 |
Late July |
May 16 |
Late July |
|
June 14 |
Aug |
June 4 |
Late July-early Aug |
Yield
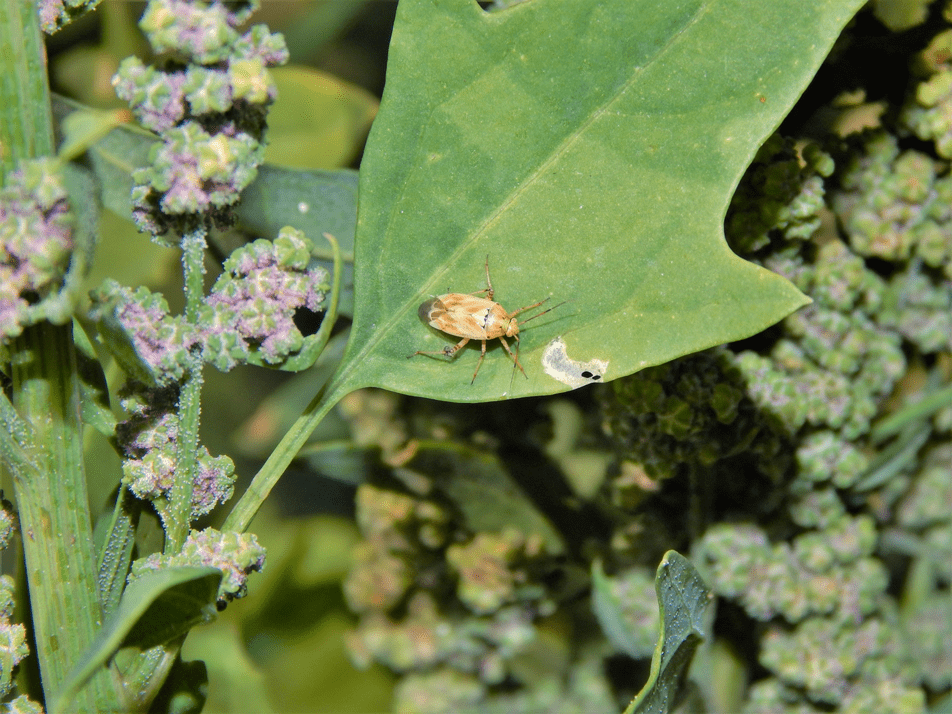
Achieving consistent yields in quinoa production has been the biggest challenge for growers. Some regions like the Salt Lake Valley of Utah attribute low yields to hot summer temperatures (above 95°F) during flowering and seed set (Buckland et al., 2018; Buckland et al., 2019). Regions like western Oregon struggle with insect pests that feed on developing seeds (Picture 5). Large commercial operations have reported acceptable yields should be above 2,000 lb/acre. The timing of planting and harvest can greatly impact both crop development (discussed earlier) and seasonal peak pest populations.
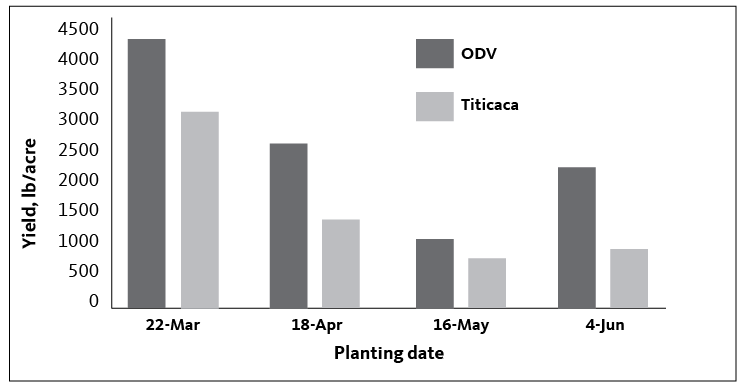
In 2018, abundant lygus bug populations decreased yield in all plots. Visual inspection of seeds showed approximately 95% of the seed on the quinoa panicles was damaged and likely not viable. Red Head, Ivory and Kaslaea varieties had the largest seed yield, and the earliest planting date had the greatest yield of the four dates. In 2019, seed set was much improved with experimental insecticidal controls, and grain was harvested from each planting date successfully. The earliest planting date again had the highest yield (Figure 1).
Figure 1 shows yields in 2019 at each planting date for two of the most commonly reported varieties throughout the U.S. Similar to 2018, early planting dates had greatest yields. Later plantings were cut and removed from the field before full seed development due to impending rains and this resulted in much lower yields. Late-season rain will often cause quinoa seeds to sprout in the head if left in the field, completely ruining yields (Picture 3). It is critical that quinoa mature and be removed from the field before fall rains begin.
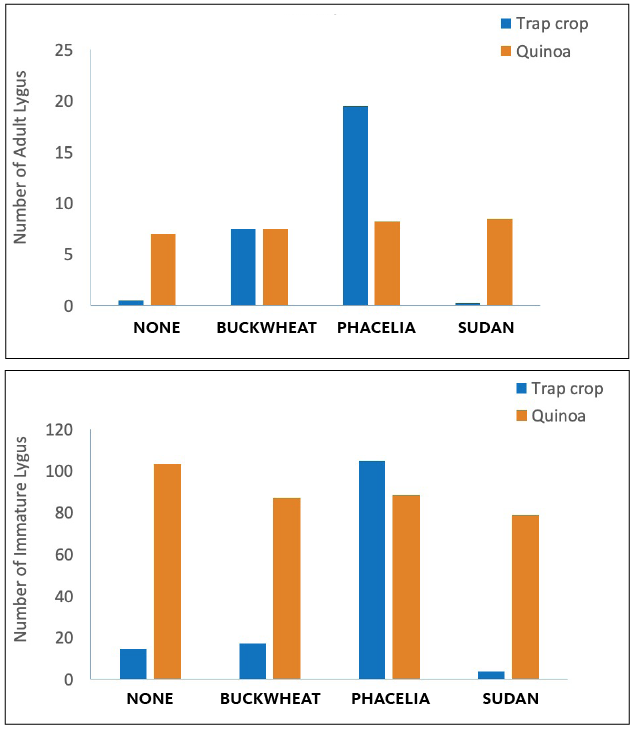
Because of the significant yield reductions due to insect damage in 2018, we planned a small lygus trap-cropping study in 2019. The study evaluated the effects of perimeter planting with potential trap crops on the lygus observed on quinoa plants (Picture 6). In this smaller study, we planted small areas of Oro de Valle bordered by one of four trap crops: lacey phacelia, sorghum-sudan grass, buckwheat or none (an untreated weedy control plot). Over three sample dates, we evaluated the incidence of lygus both within the trap crop and on quinoa. Results showed a strong preference of adult lygus for phacelia in the middle of the season, during phacelia flowering, with quinoa and buckwheat also hosting adults (Figure 2). Quinoa and lacey phacelia had the highest numbers of lygus immatures of the crops planted. Further exploration of this interaction is needed to refine systems for use at field scale.
Conclusion
Quinoa has the potential to fill some crop rotation needs for novel crops in annual cropping systems, such as vegetable or seed production. Careful planning of weed and pest control should take place prior to field selection. Planting as early as a stale seedbed can be made in the spring seems like the best practice to maximize yield and mitigate the risk of rains starting before harvest. Seed cleaning and market opportunities need to be developed further to minimize hauling costs to existing facilities in neighboring states.
References
Buckland, K.R. 2016. Increasing the sustainability of Utah farms by incorporating quinoa as a novel crop and protecting soil health. All Graduate Theses and Dissertations. 5052. https://digitalcommons.usu.edu/etd/5052.
Buckland, K.R., J.R. Reeve, J.E. Creech, and S.L. Durham. 2018. Managing soil fertility and health for quinoa production and weed control in organic systems. Soil and Tillage Research, 184, 52-61.
Buckland, K.R., J.E. Creech, G.E. Cardon, T.A. Monaco and J.R. Reeve. 2019. Quinoa response to line-source sprinkler irrigation. Journal of Crop Improvement, 33(5), 649-668.
FAOSTAT, Food and Agriculture Organization of the United States Crops and Livestock Products. Accessed Sept. 15, 2020. http://U.S..fao.org/faostat/en/#data/TP
Geerts, S., D. Raes, M. Garcia, J. Vacher, R. Mamani, J. Mendoza and C. Taboada. 2008. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). European Journal of Agronomy, 28(3), 427-436.
Hinojosa, L., J.A. González, F.H. Barrios-Masias, F. Fuentes and K.M. Murphy. 2018. Quinoa abiotic stress responses: A review. Plants, 7(4), 106.
Jacobsen, S.E. 2011. The situation for quinoa and its production in southern Bolivia: from economic success to environmental disaster. Journal of Agronomy and Crop Science 197.5: 390-399.
Livingstone, G. 2018. How quinoa is changing farmers’ lives in Peru. BBC News. Aug 18. https://U.S..bbc.com/news/world-latin-america-45008830
Murphy, K.S. and J. Matanguihan. 2015. Quinoa: Improvement and sustainable production. John Wiley & Sons.
Risi, J. and N.W. Galwey. 1991. Effects of sowing date and sowing rate on plant development and grain yield of quinoa (Chenopodium quinoa) in a temperate environment. The Journal of Agricultural Science, 117(3), 325-332.
S.K. 2016. Why the price of quinoa has fallen. The Economist. May 24. https://U.S..economist.com/the-economist-explains/2016/05/24/why-the-price-of-quinoa-has-fallen
© 2020 Oregon State University.
Extension work is a cooperative program of Oregon State University, the U.S. Department of Agriculture, and Oregon counties. Oregon State University Extension Service offers educational programs, activities, and materials without discrimination on the basis of race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, familial/parental status, income derived from a public assistance program, political beliefs, genetic information, veteran’s status, reprisal or retaliation for prior civil rights activity. (Not all prohibited bases apply to all programs.) Oregon State University Extension Service is an AA/EOE/Veterans/Disabled.