The cereal leaf beetle, Oulema melanopus L. (Coleoptera: Chrysomelidae) (Figure 1), is native to Europe and Asia, where it is widely distributed and is considered an occasional pest of small grains. Its first U.S. detection was observed in Michigan in 1962, and it has since spread throughout North America. In the Pacific Northwest, it is a pest of economic concern to cereal grains, grass forage and grass seed crops, and other grass-host species.
In the early 2000s, scientists tested the release of a small larval parasitoid wasp, Tetrastichus julis (Figure 2). The wasp, approximately 1/8 inch in size, was found to be an effective biocontrol agent of CLB populations in the Pacific Northwest. Surveys conducted from 2010 to 2014 found successful overwinter recovery of T. julis and consistently high parasitism rates of CLB larvae (greater than 80%) from commercial fields in northeastern and Western Oregon.
However, in the last few years, CLB numbers have risen, and it appears that previously effective biological control regimes have been disrupted. Some have speculated that the life cycles of CLB and its parasitoids are moving out of sync due to the warming climate.
This publication covers CLB biology, damage, integrated pest management options and current biocontrol status to reduce its impact on cereal crops.
Biology and life history
CLB has one generation per year. Adult beetles overwinter in protected sites such as grain stubble, grass crowns, areas with permanent vegetation and other sheltered places in nearby grain production fields. In spring, adults emerge when air temperatures are above 50° F and feed on available host grasses and cereal grains. Typically, overwintering adults emerge in early to mid-April in Western Oregon and late April to early May in Eastern Oregon. To estimate the CLB adult emergence and potential larval damage for your particular location, refer to the degree-day and phenology model information for this insect pest.
Adults are active for approximately six weeks, during which time they mate and lay eggs in spring-planted cereal grains (preferably) and in fall-planted cereal grains. Adult activity increases on calm, sunny days. Female CLB adults begin to lay eggs about two weeks after emergence. They can lay up to 300 eggs over a six-week period. The time required for hatching depends on temperature; eggs can hatch within four to 23 days.
Larvae feed on the host plant for three to four weeks, during which time they go through four instar stages. An estimated 90% of feeding occurs during the last two instar stages. Mature larvae drop to the ground in late June, pupate in earthen cells within the top 5 cm (2 inches) of the soil, and emerge as adults two to three weeks later in July.
After summer aestivation (the dormant stage during high heat periods), adult CLB move to any available grass hosts, such as corn or grass seed crops, to feed for two to three weeks prior to dispersal to overwintering sites, where they remain inactive until next spring. Up to 70% mortality can occur during the overwintering phase due to extreme temperatures and encounters with natural enemies.
Host range and feeding preferences
CLB larvae and adults feed solely on the leaves of cereal crops and cultivated or wild grasses, with a preference for oats, barley and wheat. Other crop and weed grass hosts include corn, sorghum, millet, rice, triticale, quackgrass, wild oats, brome and foxtail. Other CLB hosts include cultivated forage and grass seed crops such as timothy, orchardgrass, perennial or annual ryegrass, tall fescue, Kentucky bluegrass and fine fescue. Neither larvae nor adults feed on seed or grain kernels or broadleaf plants. CLB prefers spring-planted grains — oats in particular. When available, the insect will move to successively younger crops. CLB adults have been found in harvested grain; however, they can survive no more than 14 days in a grain bin.
In the Pacific Northwest, adult CLB will feed and lay eggs in several grass seed crops, but few adults are attracted to narrow-leaved grasses such as Kentucky bluegrass and fine fescue. Overwintering adult CLB are more attracted to spring-planted cereal grains compared to new stands of grass seed crops. Conversely, in summer, newly emerged adult CLB are attracted to all grass seed crops except fine fescue. Fall-planted grasses also attracted overwintering CLB adults, but damage was insignificant. However, new spring-planted grasses are at risk for damage by summer adult CLB when cereal crops have matured and other warm-season grass hosts such as corn are not available.
Damage
Adults will chew completely through the leaf, resulting in narrow slits. However, damage from adult feeding is usually insignificant.
Larvae conspicuously feed between the veins of seedling leaves or upper new leaves on older plants, removing long strips of tissue from the upper leaf surface and leaving the translucent cuticle of the lower surface intact. Tips of damaged leaves frequently turn white, giving heavily infested fields a frost-damaged appearance (Figure 4). This feeding pattern produces a characteristic “windowpane” or “frosted” appearance and decreasing plant photosynthesis, causing losses in yield and grain quality.
Yield loss depends on infestation levels as well as the crop vigor and timing or duration of CLB infestation. Plants in tressed growing conditions or poorly developed plants may suffer more severe damage.
Cereal leaf beetle integrated pest management guidelines
Pest monitoring, correct identification and understanding of pest biology are key for CLB management and sustaining parasitoid wasp populations. Begin scouting for adult activity in the spring when air temperatures exceed 50° F for several days. To detect eggs and small larvae, scout early and frequently from the onset of favorable temperatures until heads are fully emerged. Ask these questions:
- Does the CLB population meet or exceed economic threshold levels for crop growth stage? (Tables 1–3)
- Do small larvae (first and second instar) make up the majority of the population?
- Do eggs make up only a small percentage of population (less than 50%)?
- Are parasitoid wasps active in the field and, if so, at what level?
Timing
For fall-planted grains, begin scouting when plants have two visible stem nodes. Spring grain scouting should begin soon after emergence, especially for late plantings.
Sampling technique and determination of CLB thresholds
To determine if CLB populations have reached economic threshold levels, examine a minimum of 100 tillers per field. Record the number of CLB eggs and larvae per tiller from each of 10 tillers examined at 10 different sites within the field (10 X 10 method) (Appendix, Tables 1–3). Indicate the number of eggs or larvae located on the flag leaf if present (boot stage). Scout field edges separately.
You may need to subdivide large fields for more thorough scouting. Eggs may be on leaves near the ground, so carefully examine each tiller.
Because CLB is typically distributed randomly within a field, it is possible only a portion of a field will be above the threshold. In this case, you might need to treat only part of the field and leave the remainder as refuge for the parasitoid wasp.
At boot stage in spring wheat, the threshold level may need to be reduced to 0.5 larvae per flag leaf if the crop has not developed adequate tiller density or is growing under poor conditions.
Cultural control practices can help prevent infestations. These methods include tillage, avoiding late planting, utilizing trap crops and maintaining refuge areas for beneficial insects. Plant a border strip of oats two weeks after spring wheat to serve as a trap crop management strategy for CLB. Resistant varieties of wheat and barley are available in the eastern U.S., but not for Pacific Northwest small grain production systems.
If the above thresholds are met and chemical control is needed, refer to the Pacific Northwest Insect Management Handbook.
Why is it so important to determine economic threshold levels at different stages of crop growth?
The larval stage is the primary target for insecticide application. Optimal timing is when the majority of CLB eggs have hatched and small larvae are present on foliage. Eggs are not controlled with insecticides, and adults are highly mobile and can avoid control efforts. Be aware that insecticides also kill parasitoid wasps and other natural predators that are active in the field. Applications made too early without consideration of crop growth stage, CLB infestation levels or distribution within a field will not provide effective control. Follow-up treatment may be necessary if the field becomes infested at economic levels, increasing the cost of control and the potential for yield loss.
Treatment is not needed once larvae have matured and have entered pupation in the soil. Before making an insecticide application, use the field data sheet (Appendix, tables 1–3) to determine if you need to treat the entire field, only parts of the field or not at all. Untreated areas within the field or near field borders offer refuge to parasitoids and predators (Figure 5).
How do you determine if biocontrol is still working in your field?
Growers in the Pacific Northwest can determine parasitism rate of CLB larvae either by contacting their county Extension office for assistance, or by sending 25 to 50 CLB larvae to the OSU Insect ID Clinic. Collect larvae at least 1/8 inch in length, or preferably the most mature larvae (approximately ¼ inch), by clipping off the leaf that supports the larvae and placing leaves and larvae in a plastic container. See the Insect ID Clinic for submission forms and instructions.
Current T. julis parastisim rate guidelines for applying an insecticide for CLB control are:
- If 75% or more of CLB larvae are parasitized, no chemical control is needed.
- If 40%–74% of CLB larvae are parasitized, apply a registered insecticide only to the heavily infested areas of the field (usually field borders).
- If less than 40% of CLB larvae are parastized, apply a registered insecticide to infested areas of the field if economic threshold levels are met.
Current biological control status in the Pacific Northwest
Tetrastichus julis have two generations per year, mostly aligned with CLB larvae occurrence in the field. T. julis overwinters primarily as larvae inside CLB pupa buried in the soil. The female wasp inserts its eggs into a CLB larva. Several young can complete development within the same larval host. Upon hatching, the parasitoid larvae feed within the CLB larva and slowly destroy it during pupation in the soil.
CLB control is not achieved in the current season, since CLB larvae continue to feed until pupation. However, the new summer adult population is reduced based on parasitoid wasp parasitism levels.
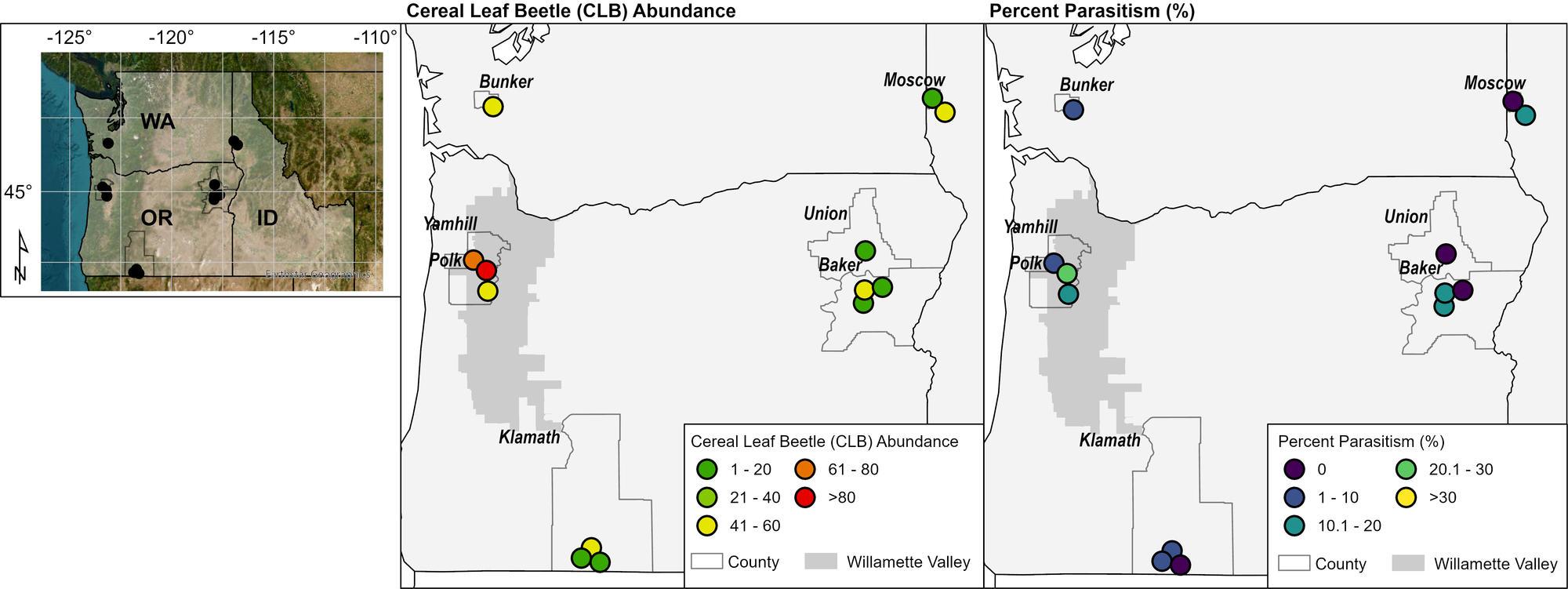
However, a recent study on climate change implications on arthropod pests and their biocontrol agents in the Pacific Northwest revealed that warmer spring conditions undercut the parasitoid’s impact on the host. CLB eggs hatched late in spring relative to the emergence and activity of parasitoid adults. The mismatch in spring development of both the CLB and parasitoid wasp, as influenced by a warming spring climate across the region, could weaken this biological control of the insect pest. A similar trend was discovered in our recent field surveys (Figure 6).
Out of the 16 commercial cereal fields sampled across the major cereal-producing regions of the Pacific Northwest during the summer of 2023, we discovered unexpectedly low parasitism levels of CLB larvae (0–28%). This may be because CLB and parasitoid wasps were active at different stages of development. Or, the low levels could be due to prophylactic insecticide applications over the last decade or so. Sporadic CLB outbreaks have been reported since 2021, underlining the importance of regular pest monitoring at different crop growth stages to maximize insecticide effectiveness and improve parasitoid survival. Using the scouting methods mentioned above and determining biocontrol status are vital to the management of CLB.
References
- Hodgson, E.W., and E.W. Evans. 2007. Cereal leaf beetle. Utah State University Extension Fact Sheet ENT-84-07PR.
- Hoffman, G.D., and S. Rao. 2006. Cereal Leaf Beetle Egg Laying on Oat Plants and Leaves off Different Ages. In Young, W.C., Ed., Seed Production Research, Oregon State University Ext/CrS 126: 39-41.
- Philips, C.R., D.A. Herbert, T.P. Kuhar, D.D. Reisig, W.E. Thomason, and S. Malone. 2011. Fifty years of CLB in the U.S.: an update on its biology, management and current research. Journal of Integrated Pest Management. 2: C1-C5.
- Rao, S., B. Quebbeman and D.L. Walenta. 2004. Host Range of Cereal Leaf Beetle. In Young, W.D., Ed., Seed Production Research, Oregon State University Ext/CrS 123: 50-51.
- Roberts, D., and D.L. Walenta. 2012. Integrated Pest Management (IPM) for the Cereal Leaf Beetle in Washington State. Washington State University Extension Service. EM054E.
- Ruppel, R.F., and F.W. Stehr, F.W. 1972 Integrated CLB control. Michigan State University Extension Bulletin E-738.
- Walenta, D.L., and S. Rao. 2011. Impact of the Cereal Leaf Beetle on Winter/Spring Wheat Yield and Economic Threshold Assessment in NE Oregon. In Young, W.C., Ed., Seed Production Research, Oregon State University Ext/CsR 136.
- Wang, H., S. Schubert, M. Suarez, J. Chen, M. Hoerling, A. Kumar, P. Pegion. 2009. Attribution of the seasonality and regionality in climate trends over the United States during 1950–2000. Journal of Climate 22: 2571–2590.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.