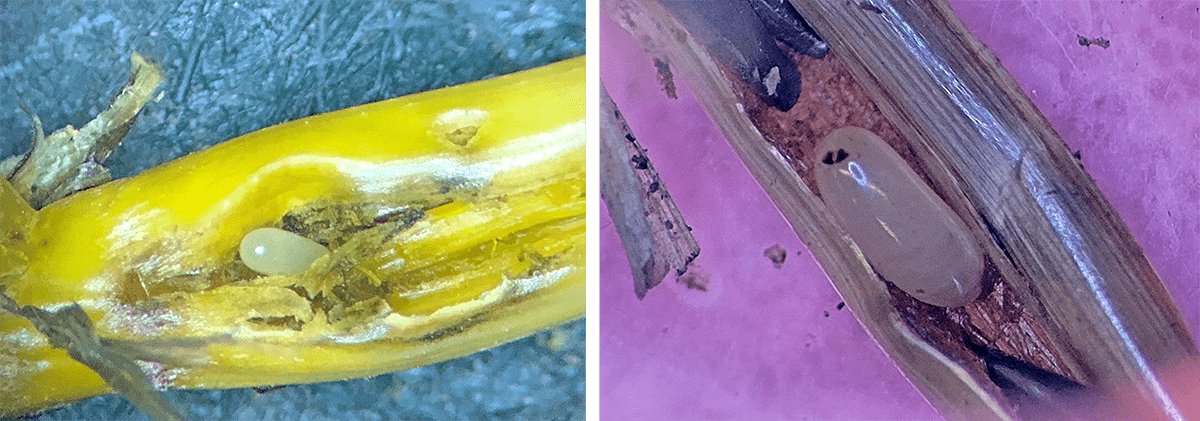
Billbugs (Sphenophorus spp.) are key insect pests in turfgrass and forage grass species across the United States. Adults (Figure 1) are beetles 0.1–0.4 inches long with a long snout or “bill” (hence the name “billbugs”). The bill carries a pair of strong jaws, or mandibles. The host range of billbugs primarily includes cool-season grasses such as Kentucky bluegrass, tall fescue and orchardgrass.
The specific host range of billbugs varies, depending on the geographic region and the species of billbug present. In Oregon, billbugs are primarily found in turf or seed production fields of Kentucky bluegrass, tall fescue and orchardgrass. Billbugs may also infest other plants, such as corn and small grains, but this has not been widely observed in Oregon.
Billbugs do most of their damage while in the larval or grub stage (Figure 2). They feed on stems, roots and crowns, causing severe discoloration and eventual plant death. Billbug management is a challenge in Oregon's grass seed production regions because multiple billbug species often coexist. Thus, life cycle differences make optimizing pest management efforts difficult. This publication describes the characteristics of billbug feeding damage, their impact on grass seed crops and effective management strategies.
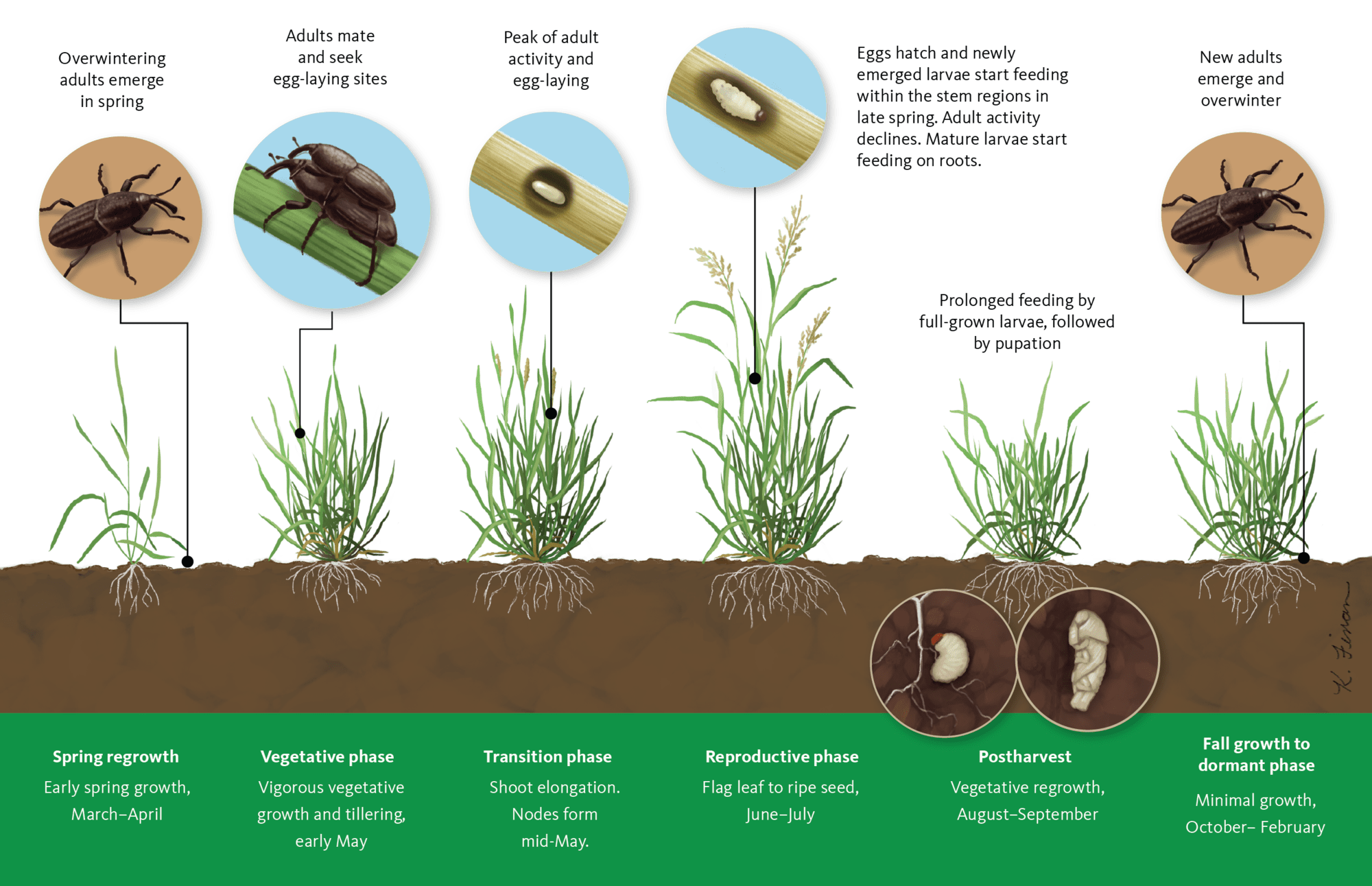
Overwintering adults resume feeding in early spring and feed on spring regrowth, chewing through young, folded leaves just above the plant crown. As leaves extend, you can see the characteristic damage of paired holes through leaf interiors (Figure 3). This should prompt you to scout the field to determine the extent of the billbug infestation.
Crop damage from adult billbugs has no effect on seed yield. However, these adults will be the source of the next generation of billbugs. When adults lay their eggs in the stems or thatch of grass, the resulting larvae feed on the roots and stems of the grass plants, damaging the crop.
The legless larvae, white to cream in color, are often referred to as grubs. Larvae bore into the crown and stems of grasses, which impairs the plants’ ability to take up water and nutrients from the soil. As larvae continue to feed, the leaves and stems of grass plants wilt, turn yellow and brown, and eventually die off. In severe billbug infestations, discolored leaves, stems or dead crowns lead to thinned areas of a grass stand, which are more visible in the fall or early spring as regrowth resumes.
Heavy larval feeding compromises the root system (Figure 4). The stems of severely damaged grasses are easily broken; it takes little effort to pull them from the soil. Root pruning can be so severe by the end of summer that crowns can be completely separated from roots with a slight tug. A tug test is frequently used to detect billbug infestation and corresponding damage. Often, sawdust-like frass is present within the crowns and in hollowed-out stems (Figure 5).
Bluegrass billbug larva can feed for up to 55 days. Larval damage during summer months likely affects the next season’s seed crop more than the current crop because of weakening or destruction of the crowns. The severity of larval feeding damage may cause either part or all of the crown to die.
Failing stands or regrowth failures are common results of increasing billbug activity (Figure 5). Adults can feed on many species of cool-season turfgrass species, but larvae are more host-specific and are only able to complete their life cycle on suitable hosts.
Billbug damage is often mistaken for drought stress or sod webworm feeding, the latter of which also create sawdust-like frass. Since billbugs are more prevalent in areas with low soil moisture, periods of moisture stress will intensify billbug damage.
Billbug species
The most common billbug species that cause economic damage to grass seed stands in Oregon include the Denver billbug (Sphenophorus cicatristriatus), orchardgrass billbug (Sphenophorus venatus confluens) and bluegrass billbug (Sphenophorus parvulus) (Figure 6). Adult billbug species are differentiated from one another by color, relative size, and the patterns and markings on their pronotum (the region directly behind the head).
The Denver billbug is larger than the other species — about 0.4–0.5 inches long with small, even indentations on the thorax.
Bluegrass billbugs are 0.2–0.3 inches long and gray to black in color, but they are sometimes coated with soil, making them appear brown or beige. The pronotum is adorned with small, evenly spaced indentations of uniform size. The rest of the body is covered with alternating rows of small and large indentations that give them a striped appearance.
Orchardgrass billbug adults are 0.3 inches long. The area behind the head is a bit broader than bluegrass billbug and covered with unevenly spaced indentations that are not uniformly sized.
The distribution of two predominant species, bluegrass billbug and Denver billbug, in grass seed crops grown east and west of Cascade Mountains overlaps significantly, while orchardgrass billbug is only known to occur in orchardgrass and tall fescue seed production systems of Western Oregon. Both bluegrass and orchardgrass billbugs are known to complete one generation per year. In contrast, Denver billbugs can complete a second generation or have a partial second generation if the winters are mild.
Billbug biology and life history
Billbugs have complete metamorphosis and have four life stages (egg-larva-pupa-adult). Monitoring efforts in commercial grass seed fields in Oregon indicate that bluegrass billbug and orchardgrass billbug complete one generation per year (Figure 9). Denver billbug has a partial second generation, where mature larvae can overwinter along with the adults. Adult billbugs overwinter in the top 2–4 inches of plant debris on the soil surface or in crowns of grass seed crop species. Adult activity does not begin until early spring, even though green plant material is available during winter months.
Billbug monitoring
Adults
Linear pitfall traps capture adult activity throughout the growing season. The trap consists of a linear PVC pipe (about 3–3.5 feet long and 2 inches in diameter) with a slit cut (0.4 inches) in it lengthwise. The pipe is buried across crop rows with a slit at the soil surface so that billbugs walking along the soil surface will fall through the slit and be caught in the trap. We recommend filling any gaps along the edges of the pipe with soil so that billbugs can easily walk onto the pipe and fall into the slit. One end of the pipe is capped, while the other is attached to an underground collection cup (Figure 10).
Frequent collections from these traps can also provide a systematic way to monitor the change in billbug activity from week to week.
Larvae
You can use sod samples (up to 12 inches in diameter and 4 inches deep) to determine larval feeding in the crown region during late summer or early fall. You can manually dissect tillers and roots in the field for signs of larvae.
Or, you can use Berlese funnels to process sod samples. Berlese funnels extract insects and other arthropods from soil and litter samples. Insects and other arthropods that live in soil will move away from a heat source, drying out the soil. A heat source above the soil samples causes the insects to move down, where they fall through a screen holding the sod sample, down a funnel and into a container for collection.
Management guidelines
Billbugs are difficult to manage effectively because of their prolonged activity during a growing season and the larval stages' inconspicuous soil- and stem-dwelling nature. Additionally, limited information exists on biocontrol options for billbug management in grass seed production systems. Generalist predators such as ground beetles are often present as a bycatch in the linear pitfall traps deployed in grass seed fields. Still, their potential to provide effective biocontrol is unknown.
Billbug species' seasonal phenology is important in timing various insecticides registered for grass seed crops (Table 1). Chemical control of billbugs in Oregon grass seed production systems mainly consists of contact insecticides, particularly Group 3A insecticides (various commercial formulations of bifenthrin or lambda-cyhalothrin) targeting adults using spring and fall applications. However, previous research on orchardgrass billbug management in Western Oregon suggested that this might not be the best approach. Adults are highly mobile, and these insecticides have short residuals, providing limited capacity to suppress any additional in-migrating adults. Increased vegetation due to active growth in the spring can also pose difficulties for insecticide penetration through the canopy and into the plant, hindering the billbug's exposure. In addition, heavy reliance on broad-spectrum insecticides in spring often results in nontarget effects on populations of natural enemies — beneficial species like pollinators or predators.
On the other hand, using contact insecticides in early fall presents a great opportunity to target new-generation adults before their overwintering. Fall management timing using bifenthrin provided up to 90% control of orchardgrass billbug in orchardgrass systems. Active movement of dispersing adults and limited crop biomass facilitates spray coverage and improves efficacy. Therefore, a fall application is warranted to target new adults before they go to overwintering sites.
For a preventive approach to be practical, target early-stage larvae in spring. Apply systemic insecticides before egg hatch to allow the product adequate time to translocate throughout the plant before feeding. Turfgrass literature recommends that systemic insecticides be applied for effective larval control when 30% of the adult population has emerged. Similarly, for a curative approach to be successful, use systemic insecticides before 50% adult emergence occurs. After this point, the larvae descend to the soil or thatch layer, making suppression difficult.
In grass seed crops, choose insecticides containing anthranilic diamides (IRAC group 28) for spring management. Chlorantraniliprole (Vantacor®) and a premix of chlorantraniliprole+lambda-cyhalothrin (Besiege®) can target billbug larvae developing within the stems or feeding on the roots. These products are more compatible with predators than the Group 3A products traditionally used at this timing, and also provide much longer-lasting residual activity.
| Trade name | Active ingredient | Mode of action/group |
|---|---|---|
| Baythroid XL | Cyfluthrin | 3A |
| Besiege | Chlorantraniliprole +lambda-cyhalothrin | 3A, 28 |
| Brigade2EC | Bifenthrin | 3A |
| Entrust* | Spinosad | 5A |
| Malathion* | Organophosphate | 1B |
| Mustang Maxx * | Zeta-cypermethrin | 3A |
| Sevin* | Carbaryl | 1A |
| Vantacor | Chlorantraniliprole | 28 |
| Warrior | Lambda-cyhalothrin | 3A |
*Billbug efficacy data not available.
Disclaimer: See specific label directions for application restrictions related to the use of straw/hay/seed screenings for livestock purposes.
Future directions
Insect phenology or predictive degree-day models used in conjunction with regular field monitoring allow early detection and informed management decisions. Efforts are underway to use billbug biology and ecology data specific to the Northwest to develop decision support tools, such as life cycle models for Oregon. Research will continue to assess the impact of existing predators such as ground beetles, entomopathogenic fungi and nematodes on billbug control. Identification of effective cultural control methods, such as resistant cultivars, is key to improving an integrated approach to long-term, sustainable pest management.
References
- Dupuy, M.M. and R.A. Ramirez. 2016. Biology and management of billbugs (Coleoptera: Curculionidae) in turfgrass. Journal of Integrated Pest Management, 7:1-6.
- Kaur, N., B.C. Donovan, L.G. Van Slambrook, D.L. Walenta and N.P. Anderson. 2021. Understanding billbug species complex in grass seed production systems in western Oregon. In Anderson et al. (ed.) 2020 Seed Production Research at Oregon State University Ext/CrS 164: 34-36.
- Rondon, S. I. and D.L. Walenta. 2011. Elucidating the biology of the bluegrass and Denver billbugs in NE Oregon. In Young III, W.C. (ed.) 2010 Seed Production Research Report at Oregon State University, USDA-ARS Cooperating. Department of Crop and Soil Science Ext/CrS 130: 59-61.
- Umble, J., G. Fisher and S. Rao. 2005. Sampling methods and seasonal phenology of Sphenophorus venatus confluens Chittenden (Coleoptera: Curculionidae) in orchardgrass (Dactylis glomerata L.). Journal of Agricultural and Urban Entomology 22: 79-85.
- Walenta, D.L., S. Rao, C.R. McNeal, B.M. Quebbeman and G.C. Fisher. 2004. Sphenophorus spp., A Complex Billbug Community Infesting Kentucky Bluegrass Seed Fields in the Grande Ronde Valley of Northeastern Oregon. In Young III, W.C. (ed.) 2003 Seed Production Research Report at Oregon State University, USDA-ARS Cooperating. Department of Crop and Soil Science Ext/CrS 124: 53-56.
- Watschke, T.L., P.H. Dernoeden and D.J. Shetlar. 2013. Managing Turfgrass Pests, second edition. CRC Press, Boca Raton, FL.
- Wickwar, D. and R. Ramirez. Billbugs in Turfgrass (Sphenophorus spp.) (2022). All Current Publications. Paper 861.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.
Trade-name products and services are mentioned as illustrations only. This does not mean that the Oregon State University Extension Service either endorses these products and services or intends to discriminate against products and services not mentioned.