Several species of leafhoppers (Hemiptera: Cicadellidae) feed and thrive on potato. They can cause direct damage by feeding, or indirect damage by transmitting pathogens, including viruses and phytoplasmas. Although a lot of information is available on viruses affecting potato, less information is available on phytoplasmas. This publication focuses on phytoplasmas.
Phytoplasmas are small bacterial parasites of plant phloem tissue. As obligate parasites, they cannot complete the life cycle without exploiting a suitable host. Phytoplasmas are associated with diseases of hundreds of plant species worldwide, including many economically important crops such as fruit trees and ornamental plants.
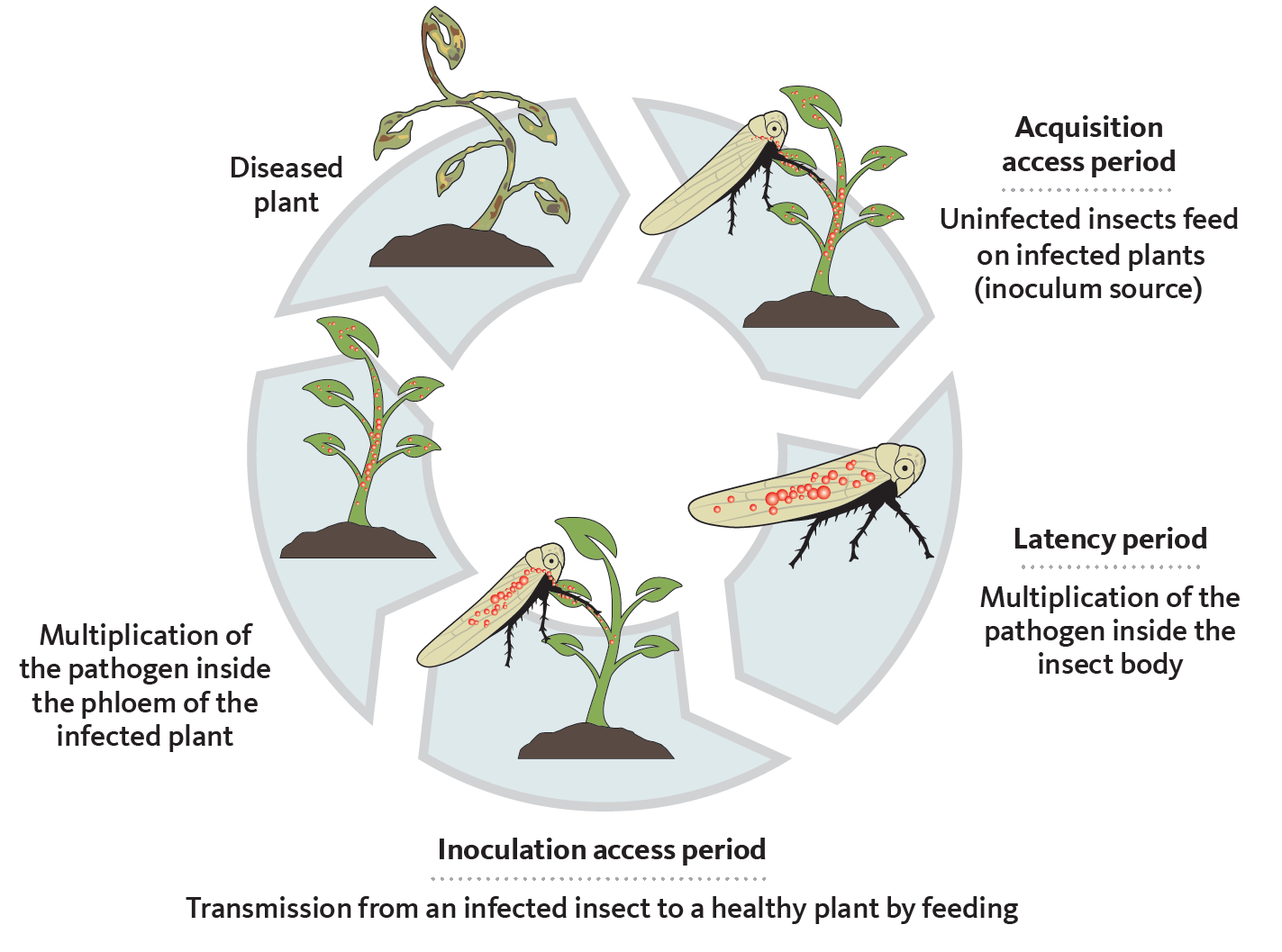
Phytoplasmas are transferred from the saliva of infected vectors such as insects into plant phloem tissues during feeding. They spread through the vascular system from the phloem. Transmission occurs in three basic steps:
- The acquisition access period, which corresponds to the feeding duration necessary to acquire the phytoplasma.
- The latency period, which is the time required from initial acquisition to when successful transmission is possible.
- The inoculation access period, which corresponds to the feeding time sufficient to transmit the pathogen (Figure 1).
Vectors and phytoplasmas have a propagative and persistent relationship. “Propagative” means that the pathogen can multiply within the insect’s body, while “persistent” rmeans that the insect remains infective for life. The length of each of these steps makes a vector efficient or not.
Purple top in the Columbia Basin
Phytoplasma diseases have emerged as a serious issue in several irrigated crops in the Columbia Basin of Oregon and Washington, including potatoes, carrots, and other vegetables. The most important disease associated with phytoplasma infection in potatoes is purple top disease, or Columbia purple top disease. PTD was first described in Canada in 1933. Over 60 years later, high incidence levels were reported anecdotally in the Columbia Basin, where growers observed that seed potatoes failed to “produce normal plants, and plants that emerged died.”
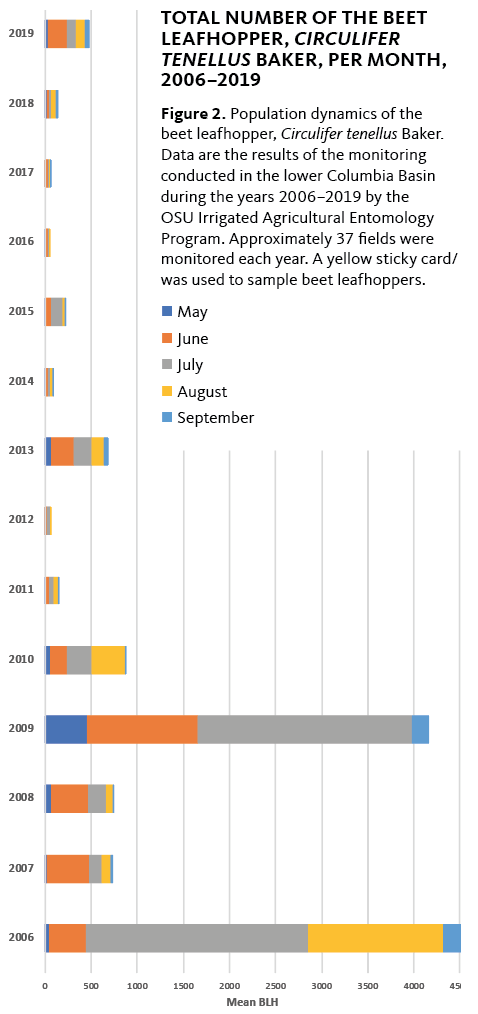
The first documented outbreak occurred during the 2002 and 2003 growing seasons, when potato growers in the Pacific Northwest experienced significant yield losses. In recent years, sporadic PTD outbreaks have been reported in the Columbia Basin of Oregon and Washington (Figure 2).
The condition was initially thought to be caused by the aster yellows phytoplasma, the pathogen responsible for PTD in other growing areas. However, researchers determined that the phytoplasma was the Beet Leafhopper Transmitted Virescence agent, or BLTV, and that the vector responsible for transmitting the pathogen was Circulifer tenellus Baker, also known as the beet leafhopper, or BLH (Figure 3).
PTD is associated with phytoplasma strains belonging to the aster yellows group (16SrI) subgroup 16SrI-B (‘Candidatus Phytoplasma asteris’), the peanut witches’-broom group (16SrII) (‘Candidatus Phytoplasma aurantifolia’), and the BLTV, which belongs to the clover proliferation phytoplasma group 16SrVI-A (‘Candidatus Phytoplasma trifolii’).
The last major outbreak of PTD in the Columbia Basin was recorded in 2013. PTD has been associated with the aster yellows phytoplasma (group 16SrI) in the north-central United States, Mexico, Bolivia and Peru. PTD has become a limiting factor for potato production in several areas of Canada, Oregon, Washington and South America. PTD disease incidence has fluctuated in the last 18 years, and long-term investigations are required to detail the epidemiology of vector and pathogen.
Beet leafhopper biology and ecology
The BLH has been identified as one of the most important pests on potatoes in the Pacific Northwest. Besides BLTVA, the BLH is also associated with beet curly top virus disease, which can cause economic losses in beans, pepper, tomato, spinach, sugar beets, melons and members of the squash family. BLHs feed and reproduce on many different plants, including sugar beet, tomato, cucurbits, spinach and weeds such as tumble mustard, pigweed, lambsquarters, groundsorel, wild radish, redstem filaree and various species of thistles.
BLH adults are small insects that measure about 3.4–3.7 mm long. They are usually whitish or greenish in color during the summer but show some dark spots dorsally in fall, becoming mostly dark in winter. The clear or dark forms are likely related to variations in climate: Lighter coloration is associated with higher temperatures during development (Figure 3, page 2).
The adults have long, slender rear legs and will jump away when disturbed. Adults move into fields in early spring in search of a suitable host and begin to feed on and lay eggs on host plants.
The eggs are whitish to yellowish in color, elongated and slightly curved. Females deposit eggs individually in the tissue of the leaves and stems. With optimal temperature conditions of 30°C, each female may deposit 300–400 eggs that hatch five to seven days later. Under temperatures of 18° C or lower, the egg incubation can last almost one month.
Newly emerged nymphs are transparent to white (Figure 4A) but become greenish in color within a few hours (Figure 4B). There are five nymphal instars, and the later instar is typically spotted with black, red and brown on the thorax and abdomen.
In Oregon and Washington, BLH completes generally three generations per year. In warmer areas such as California or Arizona, BLH can complete up to five generations.
Damage to plants and infection rate
Direct feeding by BLH causes relatively minor damage. However, BLH pest status is derived from the transmission of BLTVA. BLTVA affects primarily potatoes, beets and weeds. In general, leaves of plants infected with this phytoplasma are dwarfed, crinkled and rolled upward and inward. Veins are roughened and often swollen. In potatoes, symptoms include a rolling upward of the top leaves with yellowish, reddish or purplish discoloration; moderate proliferation of buds; shortened internodes; swollen nodes; aerial tubers; and early plant decline (Figure 5).
Molecular techniques such as polymerase chain reaction and nested PCR can detect BLTVA in both insects and plants. Both are highly sensitive methods for the detection of phytoplasmas in plant and insect DNA. Molecular phytoplasma testing results indicated that BLTVA incidence in plants and BLH varies. For instance, from 2005 to 2007, BLTVA presence in BLH collected from potatoes ranged from 9.2% to 34.8%. Similarly, BLTVA incidence in BLH collected from weeds ranged from 5.6% to 28.3%. BLTVA incidence in BLH collected from colonies where BLH were fed only with infected BLTVA plants ranged from 39.3% to 44.5%.
Integrated pest management
Protecting crops — especially early in the season — is essential from the perspective of season-long sustainability, since it is easier to control few insects rather than plenty of them. Researchers have estimated that the severity of yield loss changes in relation to BLH density, reaching an average of 15% if five insects are present on the plant (Table 1).
| BLH/plant | Average yield loss (%) | ||
|---|---|---|---|
| Low | Medium | High | |
| 1 | 3 | - | - |
| 2 | - | 12 | - |
| 5 | - | - | 15 |
Fortunately, BLHs are relatively easy to control. However, extensive reliance on insecticides can cause secondary problems, including loss of natural enemies, spikes of other pests, ecological imbalance, worker overexposure and a cascade of ecological effects. For these reasons, producers need a comprehensive plan to tackle both vector and pathogen, including monitoring, cultural, biological and chemical control.
Monitoring
Researchers, crop consultants and growers are monitoring the presence of BLH in and near potato areas. Growers in areas that could be affected by this insect are encouraged to monitor BLH numbers using yellow sticky cards. There are many different types of sticky cards, so carefully consider the choice of type, size and color intensity. Place at least one yellow sticky card 1.5–3.05 m (5–10 feet) from the edge of each potato field at least two weeks after planting. We recommend changing sticky cards weekly (Figure 6).
An interactive web map documents weekly distribution and spread of BLH in the Columbia Basin of Oregon.
Treatment threshold levels have not been established in the Pacific Norwest. BLH counts vary greatly from field to field and from area to area. For this reason, thorough coverage of the area is recommended. Researchers have suggested several general economic thresholds. According to current IPM guidelines in the Pacific Northwest Insect Management Handbook, control measures may be necessary if weekly sticky cards have between 40 and 100 BLH. In a three-year controlled study conducted in Hermiston, Oregon, results from trials using small screen cages inside a screen house indicated that 1–2 BLH per plant might be a suitable treatment threshold for the Columbia Basin. Still, producers should base insect management decisions on information specific to their fields.
Cultural control
Producers can adopt some cultural methods to reduce BLH infestations, including weed control. Although researchers have not estimated the real impact of removing weeds in or around potato fields, controlling preferred weed hosts may reduce BLH populations and BLTVA incidence. Control weeds such as kochia, Russian thistle, tumble mustard and redstem filaree. These weeds are known to host BLHs in large numbers.
Because many of the potato common cultivars in the lower Columbia Basin are highly susceptible to BLTVA, growers should select tolerant cultivars if available. Susceptible cultivars include ‘Russet Norkotah’, ‘Ranger Russet’ and ‘Umatilla Russet’. Common cultivars such as ‘Alturas’ and ‘Shepody’ are only moderately susceptible. ‘Russet Burbank’ is a cultivar that shows a reasonable amount of disease tolerance or resistance.
Biological control
To our knowledge, BLH natural enemies have yet to be surveyed and characterized in the Columbia Basin. Published information indicates that BLH eggs can be parasitized by several species of parasitic wasps belonging to two different families: Mymaridae and Trichogrammatidae. Key species include Anagrus nigriventris Girault, Aphelinoidea zarehi Triapitsyn, A. turanica Trjapitzin and Paracentrobia sp., and P. subflava Girault. Parasitism rates with some of these wasps can reach levels of 80% to 90%; unfortunately, none of them has been reported in the lower Columbia Basin. Generalist predators such as green lacewings, spiders, stilt bugs, assassin bugs and big-eyed bugs are common in potato fields, and can potentially feed on BLH.
Chemical control
The most effective management program combines chemical approaches to control the vector. A number of insecticides have proven effective. The best time to effectively control BLH is early in the season, typically the first two months after crop emergence. One foliar spray may kill BLHs in the field as well as those that arrive shortly after spraying. But studies have shown that BLH will continue to invade potato fields from surrounding areas throughout the season.
The effectiveness of chemical applications depends on many factors, including climate; availability of alternative wild hosts for vector and the phytoplasma; timing of planting or application in relation to BLH movement; the proximity of fields to BLH/phytoplasma overwintering sites; and the success of state programs to reduce BLH populations. Many years of data have shown that BLH populations vary from one location to another and from year to year. Check current products effective in controlling BLH in the Pacific Northwest Insect Management Handbook.
Conclusion
The BLH overwinters and breeds on infected weeds. During the growing season, it migrates to potato fields, where it can efficiently transmit the BLTVA pathogen. BLH acquires the pathogen by feeding on infected plants, and this pathogen is then transported by the insect vector and redeposited into healthy plants during feeding.
Infectious plants that can potentially carry BLTVA are common along many field borders and roadsides, but it is uncertain if other sources of BLTVA are present in the Columbia Basin. Once infected, BLH can carry pathogens for up to a year.
BLH is the only known vector of BLTVA, and it moves freely in the landscape. For this reason, it is important to develop a monitoring program during the growing season to measure and predict the potential threat of BLH in the fields. Start monitoring at least two weeks after planting.
Researchers should continue to investigate this three-way system — pathogen-plant-vector — in order to understand the biological and ecological factors related to sudden outbreaks.
References
- Al-wahaibi, A.K., and G.P. Walker. 2000a. Oviposition behavior of Anagrus nigriventris, an egg parasitoid of beet leafhopper, Circulifer tenellus. BioControl 45: 139–153.
- Al-Wahaibi, A.K., and G.P. Walker. 2000b. Searching and oviposition behavior of a mymarid egg parasitoid, Anagrus nigriventris, on five host plant species of its leafhopper host, Circulifer tenellus. Entomologia Experimentalis et Applicata 96: 9–25.
- Banttari, E., P. Ellis, and S. Khurana. 1993. Management of Diseases Caused by Viruses and Virus Like Pathogens. Potato Health Management, Rowe, RC. APS Press, St. Paul.
- Bayoun, I.M., G.P. Walker and S. V. Triapitsyn. 2008. Parasitization of beet leafhopper eggs, Circulifer tenellus, in California. Journal of Applied Entomology 132: 412–424.
- Bennett, C. W. 1971. The curly top disease of sugarbeet and other plants. American Phytopathology Society 7: 1–81.
- Capinera, J. 2001. Handbook of Vegetable Pests, Elsevier.
- Cook, W. C. 1967. Life history, host plants, and migrations of the beet leafhopper in the western United States, Technical Bulletin No. 1365, Agricultural Research Service, U.S. Department of Agriculture, Washington, D.C.
- Crosslin, J.M., G.J. Vandemark, and J.E. Munyaneza. 2006. Development of a real-time, quantitative PCR for detection of the Columbia Basin potato purple top phytoplasma in plants and beet leafhoppers. Plant Disease 90: 663–667.
- Crosslin, J. M., S. I. Rondon, and P. B. Hamm. 2012. Population dynamics of the beet leafhopper in northeastern Oregon and incidence of the beet leafhopper-transmitted virescence agent phytoplasma. American Journal of Potato Research 89: 82–88.
- Crosslin, J.M., J.E. Munyaneza, A. Jensen, and P.B. Hamm. 2005. Association of beet leafhopper (Hemiptera: Cicadelloidae) with a clover proliferation group phytoplasma in Columbia Basin of Washington and Oregon. Journal of Economic Entomology 98: 279-283.
- Flock, R A., R.L. Doutt, R.C. Dickson, and E.F.J. Laird. 1962. A survey of beet leafhopper egg parasites in the Imperial Valley of California. Journal of Economic Entomology 55: 277–281.
- Golino, D.A., G.N. Oldfield and D.J. Gumpf. 1989. Experimental hosts of the beet leafhopper-transmitted virescence agent. Plant Disease 73: 850–854.
- Gray, S.M., and A.G. Power. 2018. Anthropogenic influences on emergence of vector-borne plant viruses: the persistent problem of Potato virus Y. Current Opinion in Virology 33: 177–183.
- Hagel, G., B. Landis and M. Ahrens. 1973. Aster leafhopper: source of infestation, host plant preference, and dispersal. Journal of Economic Entomology 66: 877–881.
- Hamm, P.B., J.M. Crosslin, G. Pelter, and A. Jensen. 2003. Potato purple top or psyllid yellows: what was the problem in 2002, and how might it be controlled? Potato Progress 3: 1–3.
- Harries, F.H., and J.R. Douglass. 1948. Bionomic studies on the beet leafhopper. Ecological Monographs 18: 45–79.
- Hills, O.A. 1937. The beet leafhopper in the central Columbia River breeding area. Journal of Agricultural Research 55: 21–31.
- Hodgetts, J., N. Boonham, R. Mumford, N. Harrison, and M. Dickinson. 2008. Phytoplasma phylogenetics based on analysis of secA and 23S rRNA gene sequences for improved resolution of candidate species of ‘Candidatus Phytoplasma’. International Journal of Systematic and Evolutionary Microbiology 58: 1826–1837.
- Huffaker, C.B., J.K. Holloway, R.L. Doutt and G.L. Finney. 1954. Introduction of egg parasites of the beet leafhopper. Journal of Economic Entomology 47: 785–789.
- Jones, P., Y. Arocha, O. Antesana, E. Montellano and P. Franco. 2005. ‘Brotes grandes’ (big bud) of potato: a new disease associated with a 16SrI-B subgroup phytoplasma in Bolivia. Plant Pathology 54.
- Lee, I.-M., R.E. Davis and D.E. Gundersen-Rindal. 2000. Phytoplasma: phytopathogenic mollicutes. Annual Review of Microbiology 54: 221–255.
- Lee, I.-M., K.D. Bottner, J.E. Munyaneza, G.A. Secor and N. C. Gudmestad. 2004. Clover proliferation group (16SrVI) subgroup A (16SrVi-A) phytoplasma is a probable causal agent of potato purple top disease in Washington and Oregon. Plant Disease 88: 429.
- Lee, M., K.D. Bottner, G. Secor and V. Rivera-Varas. 2006. ‘Candidatus Phytoplasma americanum’, a phytoplasma associated with a potato purple top wilt disease complex. International Journal of Systematic and Evolutionary Microbiology 56: 1593–1597.
- Leyva-López, N.E., J.C. Ochoa-Sánchez, D.S. Leal-Klevezas and J.P. Martínez-Soriano. 2002. Multiple phytoplasmas associated with potato diseases in Mexico. Canadian Journal of Microbiology 48: 1062–1068.
- Meyerdirk, D.E., and N.A. Hessein. 1985. Population dynamics of the beet leafhopper, Circulifer tenellus (Baker), and asssociated Empoasca spp. (Homoptera: Cicadellidae) and their egg parasitoids on sugar beets in southern California. Journal of Economic Entomology 78: 346–353.
- Meyerdirk, D.E., and M.S. Moratorio. 1987. Seasonal population density of Anagrus giraulti (Hymenoptera: Mymaridae), an egg parasitoid of Circulifer tenellus and Empoasca spp. (Homoptera: Cicadellidae). Journal of Economic Entomology 80: 362–365.
- Munyaneza, J.E., and D.C. Henne. 2013. Leafhopper and psyllid pests of potatoes. In A. Alyokhin, C. Vincent and P. Giordanengo [eds.], Insect Pests of Potato, Global Perspectives on Biology and Management. Academic Press, Elsevier, Waltham, Massachusetts.
- Munyaneza, J.E., J.M. Crosslin and J.E. Upton. 2006. Beet leafhopper (Hemiptera: Cicadellidae) transmits the Columbia Basin potato purple top phytoplasma to potatoes, beets and weeds. Journal of Economic Entomology 99: 268–272.
- Munyaneza, J.E., J.M. Crosslin and J. Buchman. 2009. Susceptability of different potato cultivars to purple top disease. American Journal of Potato Research 86: 499–503.
- Munyaneza, J.E., J.M. Crosslin, J.E. Upton and J. Buchman. 2010a. Incidence of the beet leafhopper-transmitted virescence agent phytoplasma in local populations of the beet leafhopper, Circulifer tenellus, in Washington State. Journal of Insect Science 10: 1-10.
- Munyaneza, J.E., J.M. Crosslin, J. Buchman and V.G. Sengoda. 2010b. Susceptibility of different potato plant growth stages to purple top disease. American Journal of Potato Research 87: 60–66.
- Murphy, A.F., S.I. Rondon, R. Marchosky, J. Buchman, and J. Munyaneza. 2014. Evaluation of beet leafhopper transmitted virescence agent damage in the Columbia Basin. American Journal of Potato Research 91: 101–108.
- Nasir, M.M., S.M. Mughal and S.M. Khan. 2007. Occurrence, distribution and detection of potato purple top phytoplasma disease in the Punjab (Pakistan). Bulletin of Insectology 60: 377.
- Rondon, S. I., and A.F. Murphy. 2016. Monitoring and controlling the beet leafhopper Circulifer tenellus in the Columbia Basin. American Journal of Potato Research 93: 80-85.
- Schreiber, A., A. Jensen, S.I. Rondon, E. Wenninger and S. Reitz. 2018. 2018 Integrated Pest Management Guidelines for Insects and Mites in Idaho, Oregon, and Washington Potatoes. Idaho, Oregon and Washington potato commissions.
- Severin, H.P. 1924. Natural enemies of the beet leafhopper (Eutettix tenella Baker). Journal of Economic Entomology 17: 369-377.
- Weintraub, P.G., and S. Orentein. 2004. Potential leafhopper vectors of Phytoplasma in carrots. International Journal of Tropical Insect Science 24(3): 228235.
- Weintraub, P.G., and L. Beanland. 2006. Insect vectors of phytoplasmas. Annual Review of Entomology 51: 91-111.