Soil acidification, or a decrease in soil pH, is a natural process that is accelerated by crop production practices, primarily the use of nitrogen (N) fertilizers such as urea, ammonium sulfate, or other fertilizers containing ammonium-N.
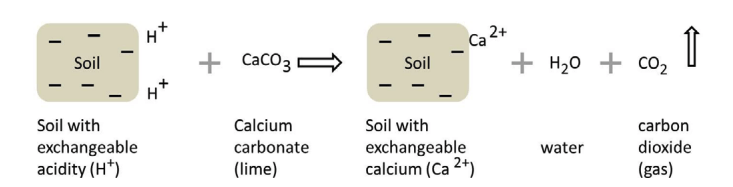
As soil acidification occurs, soil chemical and biological properties change. One chemical change is increased solubility of aluminum (Al) and manganese (Mn), both of which can be toxic to plants. Plants vary in their tolerance of Al and Mn, creating crop-specific soil pH requirements. Adding lime (Figure 1) increases soil pH (reduces acidity), adds calcium (Ca) and/or magnesium (Mg), and reduces the solubility of Al and Mn in the soil.
Publication summary
This publication describes how to estimate lime application rate and lists criteria for choosing liming materials (source), lime application method (placement), and how often to apply lime (frequency).
Lime application rate is determined using the lime requirement test (SMP buffer method). For established perennial or no-till crops, a top-dress lime application (1 to 2 t/a) may be beneficial. When very different soils are present within a field, variable-rate lime application is usually advantageous.
Liming materials vary in effectiveness. The carbonate in traditional aglime (calcium or magnesium carbonate) reacts with soil acidity to neutralize it.
Liming materials have very limited movement into the soil without incorporation. Tillage increases effectiveness of all lime materials by mixing them into the rooting zone.
Evaluate liming materials based on effectiveness (lime score) and cost. Calculate product cost per ton of 100-score lime.
Byproduct lime products can be a costeffective substitute for traditional aglime. Their characteristics should be evaluated carefully. For certified organic crops, use only lime approved by your certification agency.
Lime application method (placement) takes two forms. Lime is either applied and left on the soil surface or incorporated. In the absence of tillage, soil pH increases only in the top inch or 2 of soil since lime’s limited solubility means that the liming material must contact acidic soil before it will react and change soil pH.
Frequency of lime application is determined primarily by cation exchange capacity (CEC) and crop management practices, especially N fertilizer rate. Soil pH declines faster in sandy (low CEC) soils than in soil with moderate to high clay content. The typical rate of pH decline is approximately 0.1 pH unit per year when 100 lb ammonium N/a is applied.
For annual crop rotations, apply lime about a year before planting the crop that is most sensitive to soil acidity. For perennial crops, soil test and apply lime prior to tillage for crop establishment.
Terms used in this publication
- Ion—a molecule in which the total number of electrons is not equal to the total number of protons, giving it a net charge
- Cation—a positively charged ion
- Anion—a negatively charged ion
- N—nitrogen
- Ammoniacal N—NH4+-N
- Al—aluminum
- Mn—manganese
- Ca—calcium
- H—hydrogen
- Mg—magnesium
- K—potassium
- The cations, Al+3, Mn+2, Ca+2, Mg+2, H+, and K+ are used in this publication without charge designations except when used in chemical reactions.
- CEC—cation exchange capacity, the sum of cations electrostatically attracted to 100 grams of soil expressed in milliequivalents (meq)
- Equivalent—amount of a substance that will react with 1 gram of hydrogen
- Milliequivalent (meq)— 1/1,000 of an equivalent
- CCE—calcium carbonate equivalent
- Buffer—material that is resistant to pH change
- Slaked lime—calcium oxide that has been mixed with water, creating calcium hydroxide
- Prilled or pelleted lime—finely ground agricultural lime that has been mixed into a slurry with a water-soluble binding agent and pelletized
Soil ph management
The first step is to determine the soil pH required for your crop. The pH at which yield is diminished varies among crops. Table 1 provides minimum soil pH recommendations for western Oregon crops. Crop-specific guidance is provided in Oregon State University and Pacific Northwest Extension nutrient management guides. Other publications in this series also provide target soil pH values for many Oregon crops.
Next, use soil testing to monitor soil pH and determine whether lime application is needed to achieve your target pH. Collect soil samples at the same time each year to minimize seasonal variation. For annual crops established with tillage, monitor soil pH in the 0- to 6-inch or 0- to 8-inch depth (also the recommended depth for other routine soil analyses).
In annual crops established without tillage, and in perennial crops, soil acidity usually is greatest (pH is lowest) near the soil surface. Therefore, a separate soil sample taken from the 0- to 2-inch depth can help in estimating lime need. See OSU Extension publication EM 9014, Evaluating Soil Nutrients and pH by Depth in Situations of Limited or No Tillage in Western Oregon, for soil sampling recommendations for perennial or no-till cropping systems.
Raising soil pH with lime
Soil acidification is reversed by adding a liming material. Liming materials are oxides, hydroxides, carbonates, and silicates of Ca and/or Mg. The anion in liming materials (chemically speaking, a “base”) reacts with soil acidity (H) to neutralize it (Figure 3). The most common liming material, “aglime,” supplies carbonate as the base.
Calcium alone does not increase soil pH. For example, gypsum (calcium sulfate) and other additives contain Ca but do not contain a basic anion (carbonate, hydroxide, oxide, or silicate). Therefore, they do not neutralize soil acidity.
Liming decisions
Management decisions related to liming can be grouped into four categories:
- Lime application rate—how much lime is needed to neutralize soil acidity?
- Liming materials—what source of liming material should be used?
- Application method—how should lime be applied?
- Frequency of application—how often should lime be applied?
These decisions are discussed separately in this publication, but keep in mind that they often are interrelated.
Lime application rate
The amount of lime needed is estimated by a lime requirement test using the SMP buffer (see information below). Other lime requirement tests are offered by soil testing laboratories, but only the SMP test results have been validated by OSU Extension research for use with lime recommendation tables and figures in this publication.
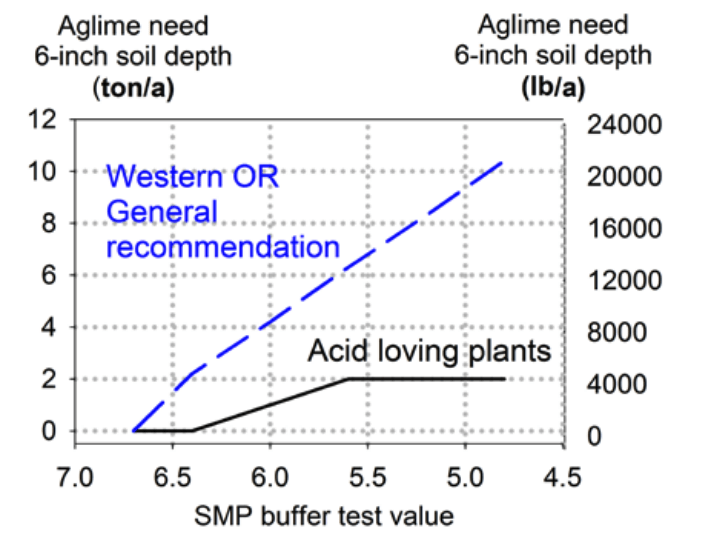
As with most soil tests, the value reported using the SMP buffer is only an index; it means nothing by itself. To interpret the SMP test for lime requirement, use either Figure 5 or Table 2. Figure 4 and Table 2 are appropriate for only a limited range of target soil pH values. Consult crop-specific OSU Extension guides for additional lime rate information.
Use Table 2 or Figure 4 is to decide the soil pH best for your cropping system (see Table 1). This pH is your “desired” or “target” soil pH. Figure 4 can be used only if your target soil pH is 6.4. Table 2 lists lime application rates needed to reach a target or desired soil pH of 5.6, 6.0, or 6.4. For acid-loving plants such as blueberry, azalea, or red maple, apply a single application of no more than 2 t lime/a, even if the SMP test indicates a greater lime need.
Using Table 2 to interpret the SMP test
Table 2 gives a recommendation for lime application (t/a) based on the soil test value you obtain from an analytical laboratory. This table is valid only when the laboratory uses the SMP lime requirement test. Other lime requirement tests have different calibration and use a different interpretive table. An example of how to use Table 2 follows.
Situation: A soil sample is collected from the 0- to 6-inch depth. The current soil pH (measured in water) is 5.0. You want to increase soil pH from 5.0 (current value) to 5.6 (desired or target value).
- Step 1. The lab analyzes the soil sample. It reports a lime requirement test (SMP) value of 6.0.
- Step 2. Find the SMP test value in the left column (blue text). For this example, find “6.0."
- Step 3. Find the appropriate column for “Desired soil pH.” In this example, the “pH 5.6” column represents the desired or target pH for your field.
- Step 4. Read “Lime to apply” (t/a) from the appropriate row and column in the table. In this example, “Lime to apply” equals 1.7 ton of 100-score lime per acre. An explanation of lime score and its use is provided later in this publication.
| Lime requirement test value (SMP) | Lime to apply (t/a) to attain desired soil pH of... | ||
|---|---|---|---|
| ph 5.6 | pH 6.0 | pH 6.4 | |
| 6.7 | 0 | 0 | 0 |
| 6.6 | 0 | 0 | 1 |
| 6.5 | 0 | 1 | 1.7 |
| 6.4 | 0 | 1.1 | 2.2 |
| 6.3 | 0 | 1.5 | 2.7 |
| 6.2 | 1 | 2 | 3.2 |
| 6.1 | 1.4 | 2.4 | 3.7 |
| 6 | 1.7 | 2.9 | 4.2 |
| 5.9 | 2.1 | 3.3 | 4.7 |
| 5.8 | 2.5 | 3.7 | 5.3 |
| 5.7 | 2.8 | 4.2 | 5.8 |
| 5.6 | 3.2 | 4.6 | 6.3 |
| 5.5 | 5.6 | 5.1 | 6.8 |
| 5.4 | 3.9 | 5.5 | 7.3 |
| 5.3 | 4.3 | 6 | 7.8 |
| 5.2 | 4.7 | 6.4 | 8.3 |
| 5.1 | 5 | 6.9 | 8.9 |
| 5 | 5.4 | 7.3 | 9.4 |
| 4.9 | 5.8 | 7.7 | 9.9 |
| 4.8 | 6.2 | 8.3 | 10.4 |
Note: "Lime to apply" values are based on application of 100-score lime and 6-inch soil sampling depth. For example, lime to apply = 1.7t/a when desired soil pH is 5.6 and the lime requirement test (SMP) value is 6.0.
Adjusting lime recommendations to account for tillage depth
Rates of lime recommended in Table 2 are based on a 6-inch depth of lime incorporation with tillage. If you plan shallow or no incorporation, such as in perennial pastures or other situations with no tillage, apply 1 to 2 t lime/a when the soil pH is below the desired level or the recommended pH for the crop. Rates higher than 2 t/a are not warranted for topdress application because the applied lime will affect pH only in the top 1 to 2 inches of soil.
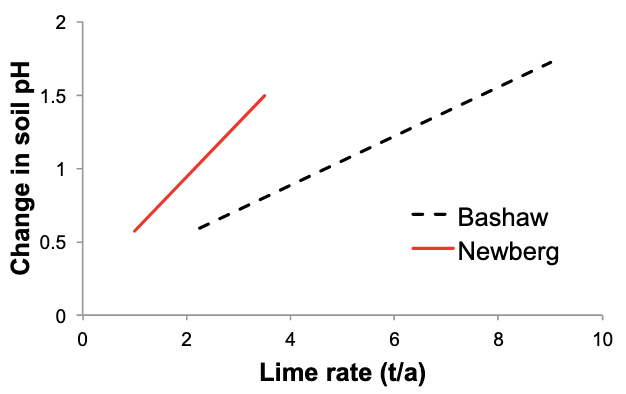
Table 3. Soil resistance to pH change (soil pH buffering capacity)
- Newberg: 2.2
- Woodburn, Chehalis, Willakenzie: 2.6–2.8
- Malabon, Dayton, Powell: 3.2–3.3
- Steiwar, Laurelwood, Cascade: 3.6–3.7
- Sauvie, Amity: 4.0–4.1
- Bashaw, McBee, Nekia, Jory: 4.4–4.6
- Salem: 5.3
Note: Table 3 is not intended to replace the SMP buffer test and should not be used to make lime rate recommendations. It is intended only to illustrate lime rate differences related to different organic matter and clay content (CEC) of various soil types. Data from Peterson, 1971.
Liming materials
After selecting a lime rate, the next decision is to choose a lime material (Table 4). For most situations, choose a product that gives the most liming value (determined by lime score) per dollar. Other factors to consider include product availability, need for Mg fertilization, desired speed of acid neutralization, and ease of application.
Liming materials are available as a powdery ground material, granule or prill, or fluid. Traditional agricultural lime (aglime, lime, ground limestone, or calcitic lime) is a finely ground material that passes through a 40-mesh sieve. It is primarily calcium carbonate.
Another mined and ground material is dolomite (dolomitic lime). In addition to calcium carbonate, it also contains magnesium carbonate. It is a common liming material for acidic soil deficient in Mg.
Calcium hydroxides and oxides (quick lime and burnt lime) are manufactured from carbonate lime. They react with acidity more rapidly than limestone, changing soil pH in days rather than weeks. Drawbacks of these products include cost and difficulty in handling and application due to their caustic nature. Their rapid reaction time also creates the potential to raise pH above the maximum value for the crop.
Granular, prilled, or pelleted lime is finely ground lime “glued” with lignosulfonate or bentonite clay, materials that slake or allow the prill to fall apart in water (soil moisture, rain, or irrigation). This material is more expensive than traditional powdered lime but provides relatively dust-free application. It is commonly used for turf and landscapes. Soil pH change may be slower for pelleted material compared to powdered lime of the same score until slaking and mixing occur.
Fluid lime, sometimes called “liquid lime,” is a suspension of very fine particles, 100-mesh or finer, mixed with water. This material has very limited solubility; for the most part, it is simply suspended in water.
Byproduct lime in Oregon usually is carbonate lime with impurities. Byproduct lime sources include paper mills, sugar beet processors, and seafood processors. Coastal areas with fisheries sometimes supply shells from shrimp and crab as liming materials (see Appendix A).
| Material | Calcium carbonate equivalent (CCE) (%) | Lime score | Ca (%) | Mg (%) |
|---|---|---|---|---|
| Common mined products | ||||
| Limestone (CaCO3) | 90 - 100 | 90 - 100 | 32 - 39 | below 1 |
| Dolomite (CaCO3 +MgCO3) | 95 - 110 | 95 - 110 | 18 - 23 | 8 - 12 |
| Specialty oxides and hydroxides | ||||
| Hydrated Lime [Ca(OH)2] | 120 - 135 | 120 - 135 | 54 | below 0.5 |
| Burnt lime or calcium oxide (CaO) | 150 -175 | 150 - 175 | 71 | 0 |
| Products | ||||
| Sugar beet lime | 70 - 75 | 40 - 50 | 25 | below 0.5 |
| Paper mill lime | 10 - 100 | 0 - 70 | 10 - 40 | below 0.5 |
| Cement plant flue dust | 110 - 120 | 105 - 115 | - | 1 - 2 |
| Shrimp and crab wastea | 30 - 40 | - | 15 - 20 | - |
| CA lime (controlled atmosphere storage) | 100 | 50 - 75 | - | - |
| Wood ash | 2 - 30 | 2 - 20 | 1 - 9 | below 1 |
a. Shrimp and crab waste CCE is expressed on a dry weight basis. Typical "as-is" moisture is 65% to 75%. Application rate of these byproducts is limited by N. A dry ton of shrimp or crab byproduct contains about 90 lb N.
Lime score
Lime score is used to adjust lime application rate based on the acid-neutralizing potential of a particular product. Lime recommendations in this and other OSU Extension publications are for 100-score lime. As lime score decreases, lime application rate must increase to obtain the same neutralizing potential as an application of 100-score material.
The quality of liming material is expressed as lime score and is defined in Oregon Administrative Rules (OAR) 603-059-0025. Lime score is a number between 0 and 125+ that combines the chemical composition (neutralizing value) or calcium carbonate equivalent (CCE), moisture (mg), and fineness or particle size (ff).
Calcium carbonate equivalent (CCE)
Effectiveness of liming materials is expressed relative to pure, dry calcium carbonate (lime score = 100). Because some liming materials are more effective than calcium carbonate per unit dry weight, they may have a lime score greater than 100. Table 4 gives CCE for common liming materials found in Oregon.
Moisture factor (mf)
This factor is a correlation for the amount of water in a liming material. Calcitic and dolomitic liming materials stored in a covered area usually contain less than 5% moisture. Some byproduct materials may contain more than 20% moisture.
Fineness factor (ff)
Fineness of a liming material is a factor governing the rate of reaction or soil pH increase. Liming materials have very low water solubility. The neutralizing reaction occurs when the surface of lime particles contacts soil and water. As the surface area of a liming material increases, so does the potential for a neutralizing reaction to occur. Fine material has a large surface area, so its rate of reaction with soil acidity is higher than that of a coarser material. Particle size determination is made for products registered by the Oregon Department of Agriculture (ODA). Coarse particles reduce lime score. (See Appendix B for ODA contact information.)
Fineness of a liming material is measured by the amount of material passing through mesh screens or sieves. The screen mesh or sieve size is the number of wires in a 1-inch length of screen. The larger the number of the mesh or sieve size, the more wires per inch, resulting in smaller holes. For example, a 20-mesh screen contains 400 openings per square inch, and the openings are 0.03 inch on a side. A 60-mesh screen contains 900 openings per square inch, and the openings are 0.0098 inch on a side
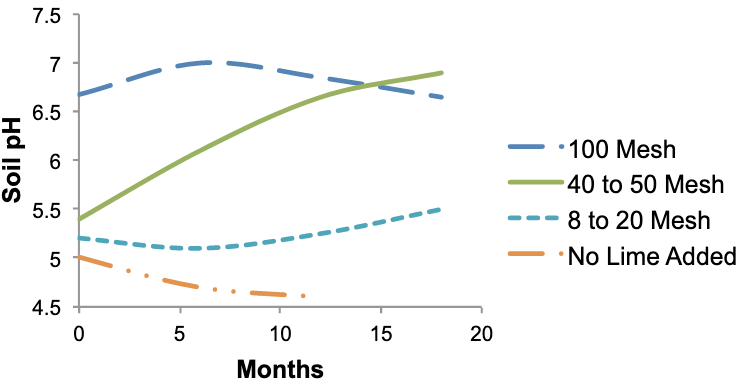
Figure 6 shows how grinding limestone affects the rate of reaction with soil acidity. When rapid pH change is needed, very fine lime (100-mesh) can change pH in a few weeks or months. The reactivity rate does not increase greatly for particle sizes smaller than 100-mesh. Many particles present in aglime are about 40- to 50-mesh. These particles require about a year for complete reaction, but a measurable change in soil pH usually occurs within a few months of application. Coarse particles (8- to 20-mesh) react very slowly.
A mix of particle size is desirable in liming materials. While fine particles are useful for rapidly increasing soil pH, coarser particles react for a year or more to maintain soil pH.
Using lime score to adjust application rates and compare costs
The lime score of a liming material is used to determine application rate, given a target application rate of 100-score lime.
Example: A paper mill lime product has lime score of 70. To deliver the equivalent of 1 t/a of 100-score lime, 1.4 t/a of paper mill lime is needed, as shown below:
Liming product rate needed:
- = (Desired rate of 100-score lime) x 100 ÷ (product lime score)
- = 1 t/a x 100 ÷ 70
- = 1.4 t paper mill lime/a
Application cost for products can be compared using lime score.
Example: An application rate of 1 t/a of 100-score lime is needed. We use the per-ton cost and the application rate to compare the per-acre cost of two materials: 100-score aglime (at a cost of $65/t) and a 70-score byproduct (at a cost of $40/t).
Cost of material for 100-score lime (1 t/a):
- = rate x cost/t
- = 1 t/a of 100-score lime x $65/t
- = $65/a
Cost of material for 70-score lime (to supply equivalent of 1 t/a of 100-score lime):
- = rate x cost/t
- = 1.4 t/a of 70-score x $40/t
- = $56/a
Fluid lime products
Fluid (liquid) lime products are marketed as alternatives to aglime. The difference between aglime (60–100 mesh) and fluid lime is that fluid lime particles are smaller. Cost per ton of material may be similar (e.g., %60/ton), but because of lower lime score (lime score = 60), the neutralizing power of a ton of fluid lime is about half that of a ton of aglime (lime score = 100).
Unlike other liquid fertilizer products such as UAN-32, which soluble, fluid lime is a suspension of fine aglime particles. Thus, unlike a soluble N fertilizer, fluid lime particles have very little ability to move into soil. Instead, they mostly stau where they were applied, usually on the soil surface.
The best use of fluid lime is pH maintenance for perennial crops where low rates of lime are needed or where traditional application equipment is difficult to use.
Description
- Finely ground carbonates that an be applied as a slurry
- Chemically identical to aglime
- Typical lime score of 60 because of water addition
Utility
- Can be applied evenly at low application rate (less than 1 t/a).
- Useful when very rapidly soil pH increase is needed near the soil surface (ie., the rooting zone).
- Can be applied more easily than aglime in nonconventional situation (e.g., orchards).
Limitations
- The cost per pound of fluid lime (based on 100-score liming material ) is higher than that of aglime.
- More frequent applications are need to maintain soil pH at the desired value.
- Fluid lime will not move into the soil with water because it is a suspension, not a solution.
Aglime vs. gypsum
Both gypsum (CaSO4) and lime (CaCO3) contain Ca; therefore, both materials add Ca to the soil. Both materials can reduce the toxic properties of soil Al. Unlike lime, however, gypsum does not neutralize soil acidity or change soil pH.
Gypsum is about 100 times more soluble than lime (Table 5). The greater solubility of gypsum allows it to move into the subsoil over the course of several years. Field trials on acidic soils with low CEC demonstrated that gypsum ameliorates the effects of subsoil acidity (e.g, reduces Al toxicity) when applied at high rates, usually 8 to 10 t/a.
Most of these studies have been conducted on highly weathered (low CEC) soils in the southeastern United States, Hawaii, and other warm or tropical climates. Treating subsoil Al toxicity with gypsum has not been evaluated in Oregon because subsoil acidity has not been a widespread limitation for crop production here.
| Material | Limestone (aglime) | Gypsum (calcium sulfate) |
|---|---|---|
| Chemical formula | CaCO3 | CaSO4•2H2O |
| Calcium (% Ca) | 40 | 23 |
| pH | 8.2 | 7.0 |
| Calcium carbonate equivalent (CCE) | 90–100 | 0 |
| Lime score | 90–100 | 0 |
| Cold water solubility (g/L) | below 0.02 | 3 |
Lime for certified organic farming
When choosing lime products for certified organic farming, consult with your organic certification agency to confirm National Organic Program (NOP) compliance. Mined agricultural limestone, dolomitic lime, oyster shell lime, and other nonsynthetic liming materials generally are allowed under NOP certification. To be an effective liming material, oyster shells must be ground to the same size as traditional mined calcitic or dolomitic limestone.
Hydrated lime or slaked lime is considered a "synthetic" liming material and is prohibited under NOP certification.
Method of lime application
Regardless of whether it is powdered, prilled, or fluid, lime is usually applied to the soil surface and either left on the surface or incorporated. In the absence of tillage, soil pH increases only in the top inch or 2 of soil since the limited solubility of lime means that the liming material must contact acidic soil before it will react and change soil pH.
Top-dressing
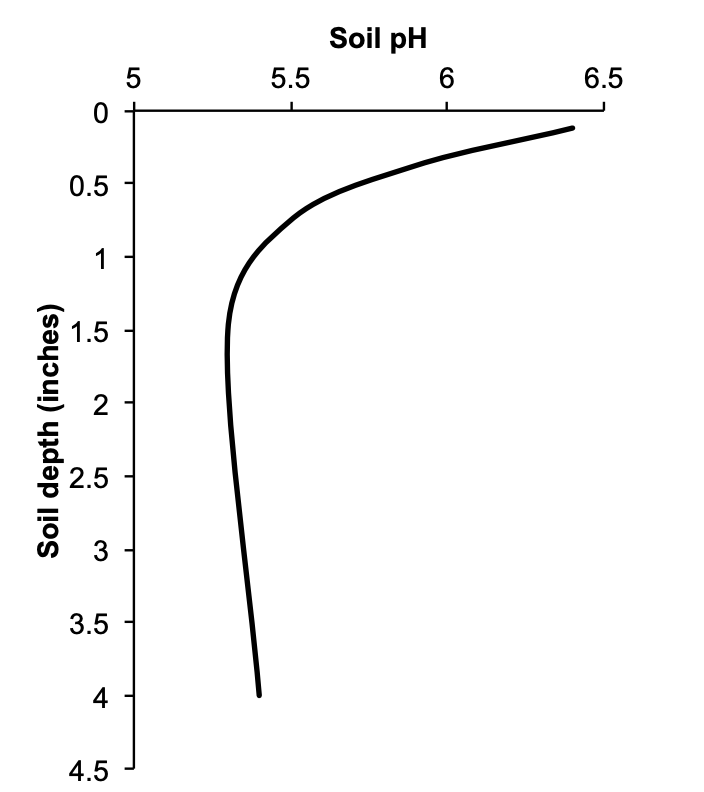
Several studies from western Oregon have illustrated the lack of soil pH change beyond the soil surface when lime is top-dressed (Figure 7). Figure 8 shows the average pH change when 1 to 1.5 t lime/a was applied to the surface of seven western Oregon perennial grass seed fields. Fifteen months after lime was applied, the soil was sampled to 4 inches. Lime increased soil pH only to a depth of one-half inch.
Top-dressing lime can be beneficial even though pH is increased only in the surface inch or 2 of soil. In many perennial cropping systems, soil pH declines in the surface 2 to 3 inches of soil after N fertilizer is applied. The typical rate of pH decline is approximately 0.1 pH unit per year when 100 lb ammonium N/a is applied. One or 2 t lime/a topdressed will increase soil pH in the surface inch of soil. The top-dressed lime helps maintain soil pH in the area where nitrification of ammoniacal N occurs. (See “Top-dress lime for forage production in western Oregon” for more information.)
Incorporation
To reduce soil acidity below 2 inches, mixing is required (Figure 9). Mixing with a disk is not as thorough as with a rototiller. Table 6 shows the results of two mixing treatments made after a 6.5 t lime/a application to a Nekia soil. Incorporation or mixing of lime with a disk resulted in an increase in soil pH to a depth of 4 inches, but primarily in the surface 2 inches. In contrast, mixing lime with a rototiller resulted in a somewhat uniform soil pH to a depth of 6 inches.
| Soil depth (inches) | Soil pH with disk incorporation method | Soil pH with rototill incorporation method |
|---|---|---|
| 0–2 | 6.6 | 6.1 |
| 2–4 | 5.6 | 6.3 |
| 4–6 | 5.3 | 5.9 |
Note: Initial soil pH was 5.3, and SMP buffer pH was 5.6. Lime was incorporated by disking or rototilling. Table by John Hart. Adapted from Doerge and Gardner, 1985a.
Banding low rates of lime with seed
Some growers of annual ryegrass seed band granular lime at planting, especially on leased ground where they are unsure of having sufficient time to obtain a return on an investment in conventional agricultural lime. While granular lime is 4 to 5 times more expensive than agricultural lime, the product is used at a rate of 100 to 150 lb/a and therefore costs 10% to 15% of a conventional 2 to 3 t/a agricultural lime application.
A low rate of banded granular lime, such as 150 lb/a, is insufficient to increase soil pH or soil Ca levels throughout the root zone. Granular lime is placed with the seed at planting to neutralize acidity in the germination zone, improve seedling growth and establishment, and ultimately help maintain seed yields on low-pH soils. Limited OSU field research shows that this practice improves yield and is a management option for field crops. See publication EM 8854, Annual Ryegrass Grown for Seed (Western Oregon) Nutrient Management Guide, for more information.
The concept of improved crop growth in acidic soils with a band application is supported by an Oregon growth chamber study (Kauffman and Gardner, 1978). In that study, lime mixed with 30% or 100% of total soil volume produced equivalent wheat yield.
Although banding of lime can improve yield, it is not recommended as a routine practice. Conventionally incorporated lime applications, while more expensive, provide greater assurance of increasing soil pH and improving seed yields on strongly acidic western Oregon soils.
Top-dress lime for forage production in western Oregon
Producers often question the effectiveness of top-dress lime on pastures. A top-dress lime application for established pastures is a prudent investment when suitable forage species are present (Figure 10). Species such as tall fescue, orchardgrass, perennial ryegrass, and clover benefit from a top-dress lime application. Pastures that consist primarily of bentgrass, velvetgrass, and similar less productive species will not increase forage yield or quality after top-dressing with lime.
Unlike fertilizer, especially N, lime is applied to maintain adequate soil pH and optimal yield rather than to increase yield. Even so, increased forage yields from top-dressed lime applications have been measured in western Oregon when the soil pH is below the crop threshold.
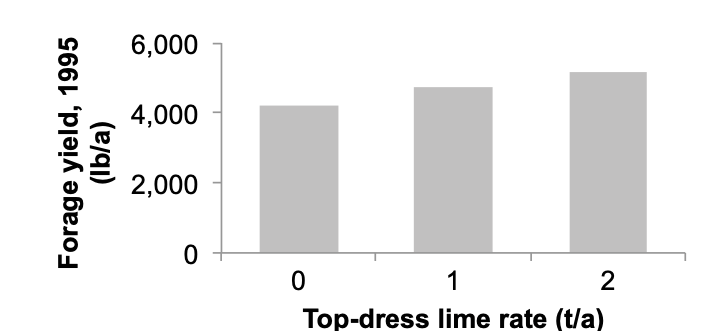
For example, a nonirrigated orchardgrass– bentgrass pasture in Tillamook County received top-dress lime applications at rates of 0, 1, and 2 t/a in the fall. The surface soil pH was 5.2, and the recommended minimum soil pH for an orchardgrass pasture is 5.8. No yield increase was measured the first spring after application. However, the second spring after lime was topdressed, the annual forage yield (sum of three clippings) increased 1,000 lb/a with the 2 t/a topdressed lime treatment compared to no lime treatment (Figure 11).
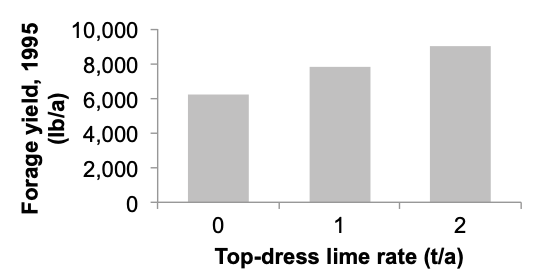
Yield increase can be substantial when the forage contains a legume and suitable grass species. For example, a nonirrigated orchardgrass–clover pasture in Lane County received top-dress lime applications of 0, 1, and 2 t/a in the fall. Similar to the pasture in Tillamook County, no yield increase was measured the following spring. However, the second spring after topdressing, annual forage yield increased 3,000 lb/a where 2 t lime/a was top-dressed compared to the treatment receiving no lime (Figure 12).
Top-dress lime rates are usually 1 to 2 t/a. They should not exceed 2 t/a. Apply lime while soils are dry, such as early to mid-fall. Before lime application, the forage in the pasture should be grazed or mowed down to a height of 3 inches. Once the lime is applied, remove livestock from the pasture for the remainder of the fall and winter.
Mixing is not always mechanical
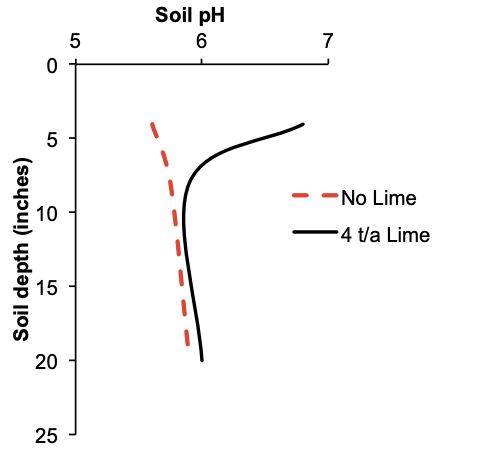
Top-dressed lime application and soil pH change were measured in a hazelnut orchard on Laurelwood soil where no tillage occurs. Soil pH and depth are noted in Figure 13. Where lime was applied, it was top-dressed at 4 t/a.
Soil pH to a depth of 4 inches increased by more than a unit when lime was applied. The undisturbed soil, leaf litter, and shade provided an excellent environment for earthworms. Openings of many earthworm burrows were observed on the orchard floor. Earthworm activity mixed lime into the soil. Below a depth of 8 inches, however, soil pH was raised only a few tenths of a unit.
Even though this example shows that earthworms can mix lime with soil, their ability to do so is not predictable. In an investigation of top-dressing lime on pastures (Rogers, 1995), top-dressed lime was mixed with the surface 3 to 4 inches of soil, presumably by earthworms, in two of four western Oregon pastures. No indicator or predictor for mixing was found. When lime is top-dressed, don’t expect earthworms to incorporate it.
Frequency of lime application
Frequency of lime application is a function of crop need and soil pH. Several factors work in concert to determine soil pH change after lime application. They include fineness of lime material, application rate, degree of mixing, soil pH, N rate, irrigation water amount and quality, residue removal, and crop rotation. Of these factors, N rate is the simplest predictor of soil pH decline, and it can be used to estimate when a lime application might be needed.
As previously stated, soil pH declines slightly less than 0.1 unit with the addition of each 100 lb N/a as urea or another ammoniacal N source on most western Oregon soils. Nitrogen is applied to wheat and grass seed at 100 to 150 lb/a. Within 3 to 5 years, these N rates will decrease soil pH 0.4 to 0.5 unit in the top 2 to 3 inches of soil.
In sandy soils with a low CEC, such as the Madras series from central Oregon, the rate of soil pH decline is about double that for surface soil from western Oregon—0.2 unit/100 lb N/a. (See “Soil CEC and liming.”)
For annual crop rotations, apply lime about a year before planting the crop that is most sensitive to soil acidity. Evaluate lime need regularly, especially when a rotation includes crops that are tolerant of acidic soil (wheat and grass seed) followed by crops that are more sensitive to soil acidity (cauliflower, spinach, red clover, and garlic).
For more information
- Annual Ryegrass Grown for Seed (Western Oregon) Nutrient Management Guide, EM 8854
- Christmas Tree Nutrient Management Guide, Western Oregon and Washington, EM 8856
- Eastern Oregon Liming Guide, EM 9060
- Evaluating Soil Nutrients and pH by Depth in Situations of Limited or No Tillage in Western Oregon, EM 9014
- Monitoring Soil Nutrients Using a Management Unit Approach, PNW 570
- Soft White Winter Wheat (Western Oregon) Nutrient Management Guide, EM 8963
- Soil Acidity in Oregon: Understanding and Using Concepts for Crop Production, EM 9061
References
Baron, L. and E. Gardner. 1975. Liming for Filbert Production in Western Oregon. Circular of Information 650. Oregon State University Agricultural Experiment Station.
Costa, R. Jr. 1978. The fertilizer value of shrimp and crab processing wastes. M.S. thesis, Oregon State University.
Doerge, T. 1985. Evaluation of long term reacidification and growth of alfalfa on selected limed soils in western Oregon. Ph.D. dissertation, Oregon State University.
Doerge, T., P. Bottomley, and E. Gardner. 1985. Molybdenum limitations to alfalfa growth and nitrogen content on a moderately acid, highphosphorus soil. Agron. J. 77(6):895–901.
Doerge, T. and E. Gardner. 1985a. Reacidification of three limed soils in central and western Oregon. In: Proceedings of the 1985 Western Oregon Fertilizer Dealers’ Conference, February 7, 1985, Albany, OR.
Doerge, T. and E. Gardner. 1985b. Reacidification of two lime amended soils in western Oregon. Soil Sci. Soc. Am. J. 49(3):680–685.
Doerge, T. and E. Gardner. 1988. Comparison of four methods for interpreting the Shoemaker-McLeanPratt (SMP) lime requirement test. Soil Sci. Soc. Am. J. 52(4):1054–1059.
Fitzgerald, M., M. Mellbye, and D. Sullivan. 1997. Liming with recycled paper residue (RPR). In: W.C. Young III (ed.). 1997 Seed Production Research at Oregon State University, USDA-ARS Cooperating. Ext/CrS 111. Oregon State University Department of Crop and Soil Science.
Hart, J. and M. Mellbye. 2010. Annual ryegrass seed production in acidic soil. In: W.C. Young III (ed.). 2009 Seed Production Research at Oregon State University, USDA-ARS Cooperating. Ext/CrS 129. Oregon State University Department of Crop and Soil Science.
Horneck, D. 1994. Nutrient management and cycling in grass seed crops. Ph.D. thesis, Oregon State University. Chapter 5 and appendix.
Jackson, T., H. Rampton, and J. McDermid. 1964. The Effect of Lime and Phosphorus on the Yield and Phosphorus Content of Legumes in Western Oregon. TB 83. Oregon State University Agricultural Experiment Station.
Jackson, T., W. Sheets, N. Mansour, and H. Mack. 1974. Lime: Response in spinach and other vegetables. Oregon Vegetable Digest 23:(2)1–2.
Kauffman, M. 1977. The effect of soil acidity and lime placement on root growth and yield of winter wheat and alfalfa. Ph.D. dissertation, Oregon State University.
Kauffman, M. and E. Gardner. 1978. Segmental liming of soil and its effect on the growth of wheat. Agron. J. 70:331–336.
Mellbye, M. 1988. The effect of surface liming on soil pH and calcium. Handout, Soil Acidity and Liming Symposium, Oregon State University, Corvallis.
Mellbye, M. 1992. Surface limed soil—six years later. OSU Extension Update (Linn County), Vol. XI, No. 9, p. 6.
Mellbye, M. 1996. Effect of recycled paper residue soil amendment (RPR) on ryegrass seed yields. In: W.C. Young III (ed.). 1996 Seed Production Research at Oregon State University, USDA-ARS Cooperating. Ext/CrS 110. Oregon State University Department of Crop and Soil Science.
Myer, R. 1999. Agricultural Use of Wood Ash in California. Publication 21573. University of California
Peterson, P. 1971. Liming requirement of selected Willamette Valley soils. M.S. thesis, Oregon State University.
Rogers, J. 1995. The effect of top-dressed lime upon pasture production and quality. M.S. thesis, Oregon State University.
Sikora, F. 2006. A buffer that mimics the SMP buffer for determining lime requirement of soil. Soil Sci. Soc. Am. J. 70:474–486.
Sikora, F. 2007. Replacing SMP Buffer with Sikora Buffer for Determining Lime Requirement of Soil. Publication 6. SERA IEG-6 (USDA-NIFA multistate group).
Stevens, G., D. Dunn, and B. Phipps. 2001. How to diagnose soil acidity and alkalinity problems in crops: A comparison of soil pH test kits. J. of Extension.
Stevens, G., A. Wrather, H. Wilson, and D. Dunn. 2002. Soil sampling fields with four types of probes. Crop Management. doi:10.1094/CM-2002-1025-01-RS
Young, W. III, M. Mellbye, G. Gingrich, T. Silberstein, T. Chastain, and J. Hart. 2000. Defining optimum nitrogen fertilization practices for grass seed production systems in the Willamette Valley. In: W.C. Young III (ed.). 2000 Seed Production Research at Oregon State University, USDA-ARS Cooperating. Ext/CrS 115. Oregon State University Department of Crop and Soil Science.
Appendix A. Byproduct liming materials: Are they a good fit for your needs?
What are byproducts?
B-product lime products are derived from industrial processes such as paper manufacturing, refining sugar beets, or processing shrimp and crab. Byproduct lime products may be sold and applied by agrichemical (fertilizer) dealers, or they may be managed by a third party land application contractor who works for the byproduct generator (factory or mill).
Compared to other lime products, particle size in byproduct lime may not be as important in determining effectiveness as a liming material. Most byproducts are chemical precipitates from industrial processes. They are not rock (limestone). Large byproduct particles usually “melt” rapidly in soil after application. Byproduct lime occasionally reacts with soil acidity faster than traditional aglime.
Byproducts can vary in quality, even among a class of materials (e.g., wood ash). Calcium carbonate equivalent (CCE) for byproducts can vary from near 10% to near 100%. Each byproduct is a “package” with a unique set of chemical and physical characteristics. Most, if not all, byproduct lime products are not eligible for use on fields certified as compliant with the USDA National Organic Program.
How are they regulated?
Byproducts are sold to farmers as a lime material registered by the Oregon Department of Agriculture (ODA), or they can be distributed directly by a factory or mill under Oregon Department of Environmental Quality (DEQ) land application permit.
Byproduct lime registered by ODA is subject to the same ODA regulations for lime score as any other lime product. ODA registration ensures a minimum lime score, and trace element concentrations in the product must not exceed ODA standards for arsenic (As), cadmium (Cd), mercury (Hg), lead (Pb) and nickel (Ni).
Byproducts applied under a DEQ land application permit require environmental testing (total metals and other contaminants), but a minimum lime score is not guaranteed. Obtaining frequent lime score analyses within a season and between byproduct “batches” is strongly recommended.
ODA registration or DEQ approval of a byproduct lime material typically does not require analysis for other elements that may have agronomic value, such as potassium (K), N, or constituents that may be important to crop/soil management, such as boron (B), organic matter (OM), carbon (C) or carbon-to-nitrogen (C:N) ratio. Byproduct vendors should provide these analyses upon request.
What do I need to know about byproduct lime?
Ask the following questions about any byproduct lime material.
Liming value
- What is the cost of the byproduct lime in terms of CCE or lime score equivalence?
Post quality
- Does the byproduct contain other plant nutrients that might provide benefit to your soils (e.g., K) or might be injurious at high rates (e.g., B)?
- Has the byproduct been used successfully locally on the kind of cropland (soil, crop rotation) that you have? Are there any reports of crop injury resulting from byproduct use?
- What product characteristic limits the desired application rate? Maximum desired soil pH? N deficiency (high C:N paper solids)? K? B? Herbicide inactivation (wood ash)?
Logistics
- How much flexibility will you have in the timing of byproduct application?
- What is the minimum application rate for the byproduct, using available equipment, and how much 100-score lime is supplied at the minimum application rate?
- What additional management practices might be necessary?
Lime byproducts in Oregon
Not all byproducts are created equal. The most common lime byproducts used in Oregon can be grouped into three general categories:
- High-CCE products derived from quick lime (calcium hydroxide)
- Wood ash
- Paper clarifier solids
High-CCE products derived from "quick lime" (calcium hydroxide) are the most useful liming byproducts. They have CCE greater than 40%. Examples include sugar beet lime and other materials derived from precipitated quick lime. Quick lime is used in industrial processes such as paper making and sugar refining to achieve a high pH (12). The recovered lime from these factory processes consists mainly of carbonates (same active ingredient as aglime).
Beet lime and other high-value byproducts are usually purchased from the factory by fertilizer dealerships and then custom applied to farm fields. These products are a reliable lime source. No significant product quality issues have been reported.
Wood ash (from hog fuel boilers or biomass-to-energy facilities) is available across the state. This product has a lime score of 2 to 20. Wood ash can vary considerably in composition and utility. Although called wood ash, it can contain residues of other materials that are burned together with forest byproducts.
Some ash acts like low-grade activated charcoal, binding soil-active herbicides and making them ineffective in killing weeds. Burn temperature affects this property, known as herbicide sorption.
Chemical composition of ash varies depending on the origin of the ash within a facility. The highest concentration of trace element contaminants such as arsenic (As) and B are usually found in fly ash (from smokestack scrubbers).
Because of the nonuniformity and unpredictability of ash characteristics, caution is warranted when using wood ash. We recommend that you obtain a complete analysis, consult with those having experience with the ash, and evaluate product performance on limited acreage before using it for routine application.
Paper clarifier solids, sometimes called “paper sludge,” are a mixture of wood fiber, carbonates derived from quick lime, and inert materials. It is collected in settling basins (clarifiers) at mills producing cardboard or other paper products. The more wood fiber present, the lower the lime score. CCE is usually 10% to 20%. The organic matter content in paper solids (cellulose) is similar to that found in straw, and paper fiber has a high C:N ratio (greater than 100:1). Herbicide sorption (from added organic matter) has not been reported following paper solids application.
The major management problem related to paper solids application is providing sufficient and properly timed N for the first crop after application. As paper fiber decomposes in soil, the microbes performing decomposition compete with the crop for N. During the first growing season after paper solids application, extra N fertilizer is needed to compensate for N consumed by the decomposition process.
Because paper fiber degrades slowly, extra N fertilizer application is usually required in both fall and spring when growing fall-seeded winter annual crops such as annual ryegrass (for seed). Even with supplemental N fertilizer application, a reduction in first-year seed yield can occur because the timing of N availability to the grass seed crop is altered by the decomposing paper solids.
Microbial activity differs between fields and crop years; therefore, the decomposition rate of paper clarifier solids varies. Furthermore, each factory source of paper solids is unique in its rate of decomposition and its need for supplemental N fertilizer.
University field research to support appropriate N fertilizer recommendations following paper clarifier solids application is generally lacking. In most cases, N fertilizer rate and timing are based on grower experience. A single-year trial on Woodburn soil, with oats, determined that 1 to 3 lb N (from fertilizer) was needed per dry ton of paper solids to compensate for N immobilization (Fasth and Karow, 1994, unpublished). In the second growing season after application, a paper solids application had no effect on grass seed crop N uptake (Fitzgerald et al., 1997).
High rates of paper solids application can be detrimental to crop establishment on poorly drained soils because the paper solids increase the waterholding capacity of soil. This problem has been most evident in fields without drainage tile where the water table is at the surface for extended periods in winter and spring.
Meadowfoam seems to be especially susceptible to stand loss in this situation. Some portions of annual ryegrass fields also had little or no seedling emergence, sometimes accompanied by a red slime on the soil surface. Red slime was an iron-reducing bacteria that flourished in anaerobic soil (without oxygen) containing large quantities of organic matter (from the paper solids).
Appendix B: State regulation of liming products
Oregon Department of Agriculture
The ODA Fertilizer Program inspects and registers lime products distributed in Oregon. Lime products must be registered with ODA before they can be distributed in Oregon. Lime products are monitored and regulated to provide:
- Uniform and accurate product labeling
- Assurance, through sampling and analysis, that products provide the nutrients and other benefits claimed
- Protection for Oregon's environment and natural resources from heavy metals and other contaminants
For questions about lime product regulation in Oregon, please contact:
Oregon Department of Agriculture Fertilizer Program
635 Capitol Street NE
Salem, OR 97301-2532
Phone: 503-986-4635
Fax: 503-986-4735
Oregon Department of Environmental Quality
In addition to ODA, lime byproducts derived from industrial manufacturing processes may be regulated by either the DEQ Solid Waste Division or the DEQ Water Quality Division. Lime byproducts derived from an industry’s wastewater treatment system (e.g., clarifier solids) are regulated by the Water Quality Division. Lime byproducts derived from other industrial processes (e.g., wood ash resulting from combustion of wood chips in a boiler) are regulated by the Solid Waste Division.
Appendix C. Consideration for soils with volcanic ash in parent material
Even though the SMP test measures many more times the H concentration than do soil pH tests, the SMP value is usually only about 0.8 unit higher than soil pH. The higher value for the SMP measurements results from the 7.5 pH of the solution.
The typical difference between soil pH and SMP buffer pH is not seen in some soils, such as the Sifton series. The difference between soil pH and SMP buffer pH for this soil can be as small as 0.2 unit, and sometimes the buffer pH is lower than soil pH. Other soil series formed in mixtures of volcanic ash, such as the Parkdale and associated soils in the Hood River Valley, may behave similarly.
The relationship between soil pH and SMP buffer pH for the Sifton soil does not indicate that the SMP test inadequately measures lime need. This soil’s mineralogy differs from other western Oregon soils.
This difference increases both buffer capacity and lime requirement. The recommended rate of lime in Table 2 is not economical. Use a lower rate of lime than that recommended by Table 2, concentrate it with limited mixing (shallow rather than deep disking), and monitor soil pH after application.
A small area of Sifton soils is found in Columbia, Multnomah, and Lane counties. The largest area, approximately 5,500 acres, is located in Marion County on high terraces in the Stayton Basin and along Mill Creek between Turner and Salem.
Sifton soils formed in gravelly alluvium containing volcanic ash in the upper part. They have a thick, black gravelly loam A horizon over dark brown gravelly loam subsoil.
Acknowledgments
We appreciate careful and thoughtful review comments for this publication from Sam Angima, Oregon State University; Gerard Birkhauser, Washington State University; Tabitha Brown, Washington State University; Craig Cogger, Washington State University; Brian Cruickshank, Wilco; William Fasth, Brown and Caldwell; Melissa Fery, Oregon State University; Matt Haynes, Oregon Department of Agriculture; Tom Miller, Fitzmaurice Fertilizer; Joe Moade, Crop Production Services; Steve Salisbury, Wilbur-Ellis Company; Eric Shumaker, Wilbur-Ellis Company; and Donald Wysocki, Oregon State University.
The authors thank Mark Mellbye for his contributions to this publication. In addition to photos, some of the data Mark collected about soil pH management in western Oregon grass seed cropping systems during his tenure as an OSU Extension agent is used in this publication.
We thank Gale Gingrich, OSU area Extension agronomist emeritus, for supplying photos.
Photos: All photos copyright Oregon State University.