Introduction
Oregon vineyard managers are increasingly concerned about vineyard viruses. The increasing incidence and spread of viruses and insect vectors (disease transmitters) throughout all key grape industries worldwide has have increased these concerns. Vineyards in California, Oregon, Washington and Idaho have battled the spread of grapevine leafroll viruses (GLRaVs). Oregon has been identified with GLRaVs and their key vectors, including several mealybug species. Grapevine viruses can decrease vine health, yield and fruit quality, and these compromise vineyard longevity and sustainability.
To prevent the movement of GLRaVs and vectors into new vineyards in the area, growers should scout their vineyards to identify GLRaVs symptoms and insect vector infestations. This publication is a field guide to identify vineyard blocks that may have mealybug and GLRaVs.
What are grapevine leafroll viruses?
Grapevine leafroll viruses are members of the Closteroviridae family, which includes 10 distinct viruses. Currently, there is no cure for vines infected by GLRaVs.
Although GLRaVs are generally found in the vascular tissues of wood (phloem), they are also systemic throughout the vine. They are often spread by cuttings, such as grafting of clean scions onto infected rootstocks or infected scions onto healthy rootstocks.
How the viruses spread
Grapevine leafroll viruses are transmitted (vectored) by several species of mealybugs (Hemiptera: Pseudococcidae) and soft-scale insects (family Coccoidea). Prominent mealybug vectors include grape mealybug (Pseudococcus maritimus), vine mealybug (Planococcus ficus), obscure mealybug (Pseudococcus viburni) and Gill’s mealybug (Ferrisia gilli). Soft-scale insects, such as the European fruit lecanium scale, cottony maple scale and wooly vine scale, have been reported as transmitters of GLRaVs. However, these soft-scale insects are less efficient at infecting vines with GLRaVs than mealybugs.
Spread to previously uninfected areas can occur rapidly when a vineyard has GLRaVs-infected vines and an insect vector. When vectors are present, vineyards with less than 10% GLRaVs infection can show greater than 90% infection within just 10 years.
The impact of mealybugs
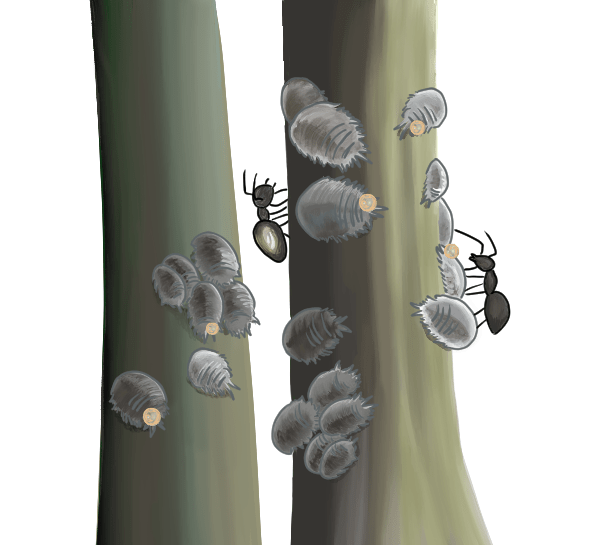
Mealybugs feed on the vascular tissue of grapevines and expel a thick, sticky, shiny substance known as “honeydew,” a byproduct of digestion. Honeydew is basically excess plant sugars (Figure 1). Ants feed on honeydew and are often associated with mealybugs.
Mealybug lifecycle
Female mealybugs undergo incomplete metamorphosis (Figure 2). Adult females are wingless, 2–5 millimeters long, oval-shaped and white with waxy secretions. Some species have thin filaments along the margin of the body. Females lay several hundred eggs in cottony sacs. Nymphs (crawlers) may hatch and emerge after 1–15 days during summer months, depending on temperature. Crawlers closely resemble adult females in body shape.
Adult male mealybugs undergo complete metamorphosis. They are tiny with long antennae and, unlike adult female mealybugs, a single pair of wings. Male mealybugs do not feed during the adult stage. They respond to a sex pheromone, a chemical signal released by mature females. Adult male mealybugs are short-lived and may die within days of emergence, depending on temperature.
Grape and obscure mealybugs can produce two to three generations each year. When gently probed, grape mealybugs secrete a reddish orange defensive fluid. Vine mealybugs can produce three to nine generations each year, depending on the temperature and environmental conditions. Vine mealybugs produce a clear defensive fluid when probed. Vine mealybugs feed on all parts of the vine, including the roots, making control difficult.
Managing the GLRaVs and mealybugs
Knowing the common signs and symptoms of mealybug GLRaVs in your vineyard will help manage these
pests before they become well-established. Early recognition of vineyard infection with GLRaVs, mealybugs or both is critical to minimizing their spread within your vineyard, into neighboring vineyards and to the industry in your region. Mealybugs are difficult to eradicate once vineyards become infested, and the GLRaVs are impossible to cure. The best defense is to train vineyard crews to identify signs and symptoms and manage these pests.
Mealybug monitoring
Mealybugs are difficult to find at low infestation levels. The most efficient way to monitor for low levels of mealybug is by using pheromone-baited traps (See Trapping and Identifying Mealybugs in Oregon Vineyards, EM 8998). Commercial pheromone traps are available for grape and vine mealybug and can determine low infestation rates. Vineyard mealybug infestation may go unnoticed for several years. Many symptoms of infestation can be found within vine canopies by peeling the bark on trunks and cordon, digging around the base of the vine trunk and sampling the root zone of the alleyways or cover crop (Figure 3).
Follow mealybug monitoring guidelines for identification and seasonal trends (Table 1, Figure 3).
| Location on plant | Signs | When to scout |
|---|---|---|
| Leaves and canopy | Various stages of mealybug development — adults, immature stages and egg sacs (Figure 1). | Midsummer to harvest |
| Leaves and canopy | Sticky honeydew and sooty mold can be found on the surface and may be associated with ants. | Late summer to harvest |
| Leaves and canopy | Vines may become weak, and basal-shoot leaves may fall prematurely with heavy mealybug infestations. | Late summer to harvest |
| Fruit clusters | Sticky clusters on vines before harvest. Look inside clusters for waxy, sticky white residue between berries and on rachis (see Figure 1). | Summer to harvest in vineyard and crush pad as well as during processing |
| Fruit clusters | Sooty mold development | Late summer to harvest |
| Trunk or cordon | Wet or shiny appearance of bark caused by honeydew. This may be accompanied by sooty mold (see Figure 1). | Summer to harvest |
| Trunk (under bark) | The majority of the mealybug populations may be found under the bark. | Year-round |
| Base of trunk and roots (below soil surface 0–6" | Adult mealybugs and immature crawlers. Waxy, fuzzy, white and cotton-like appearance. | Winter to early spring |
| Cover crop or weeds | Look for mealybugs at the shoot-root-soil interface under cover crops or weeds (Figure 3). | Late summer to fall |
| Vine debris (pruning wood, leaves, thinned fruit) | Leaves, canes and fruit may contain various stages of mealybugs as well as egg sacs. | Year-round |
Crawlers are difficult to find once they leave the egg sac. Mealybug eggs can be observed within egg sacs by using a hand lens. In the Pacific Northwest, the best time to sample for the presence of mealybugs is mid-July through October (Figure 3), when populations are actively growing. They are found in higher numbers in their preferred feeding spots during this period. Mealybugs tend to shelter under bark and in pruning wounds and crevices. Other key areas to sample include exposed plant parts: trunk, cordon, canes, shoots, leaves and clusters. Be sure to sample roots in sandy soils. Honeydew, ants and sooty mold are common signs of mealybug infestation and can be found on any part of vines. Peel bark away to look for egg sacs.
If you identify symptoms of mealybug infestation, record your observations, noting where in the vineyard they were found. To confirm the species of mealybug, collect and submit samples to the Oregon State University Insect ID Clinic. Contact your Extension horticulture agent before resorting to chemical control.
Monitoring vines for viruses
Symptoms of GLRaVs resemble general stress responses in grapevine (Table 2). Vine disease symptoms can be mistaken for other factors, such as water stress, nutrient deficiency, crown gall, vertebrate damage or mechanical injury. Symptoms vary between years and may go unnoticed. Symptoms expressed also vary with grape cultivar, age of the vineyard, stage of infection, viticulture practices, environmental conditions and the strain of the GLRaVs. Vines stressed by other biotic or abiotic factors may exhibit symptoms more readily and consistently than healthier, unstressed vines. GLRaVs-impacted vines may express symptoms or combinations of symptoms.
Laboratory tests of tissue samples are required to diagnose virus infection; visual symptoms alone are inadequate. If you suspect you may have the virus in your vineyards, note your seasonal observations. Record the block information, observation date, phenology and what you saw. Collect and submit samples for GLRaVs analysis to verify whether the symptoms are related to the virus.
The best analyses for the GLRaVs are enzyme-linked immunosorbent assays (ELISA) and polymerase chain reaction (PCR) tests. Before submitting samples, call the lab and ask which tests they offer. Autumn, during ripening through harvest or even post-harvest, is the best time to sample. Ask the testing lab to verify the appropriate tissue type, timing of sample collection and handling before analysis. Collect leaf samples (blade and petiole). Place leaves in a clear plastic zip bag with a dry paper towel and ship them overnight or drop them off at a virus testing lab. If test results are negative for GLRaVs, consider your vineyard management practices; soil or leaf tissue analysis for macro and micronutrients may be indicated, as nutrient deficiencies may cause similar symptoms.
| Location on plant | Symptoms | When to scout |
|---|---|---|
| Leaves | Discoloration, generally between veins, but may vary. | August until leaf fall |
| Leaves | Red cultivars—red to reddish purple (Figure 4) | August until leaf fall |
| Leaves | White cultivars—yellowing or chlorotic mottling (Figure 5). | August until leaf fall |
| Leaves | Leaf margins may curl downward (see Figure 4). | August until leaf fall |
| Fruit clusters* | Loose and smaller clusters. Poor fruit set and lower cluster weights and vine yield. | Fruit-set until harvest |
| Fruit clusters* | Poor color development in red cultivars. | Veraison until harvest |
| Fruit clusters* | Low ВєBrix (soluble solids). | Veraison until harvest |
| Fruit clusters* | Fruit never ripens to desirable soluble solids (ВєBrix) or titratable acidity. | Harvest |
| Vine growth | Decreasing vine growth and yield. | Year-round |
| Vine growth | Delayed bud break and shorter shoots in spring. | Early spring |
| Vine growth | Infection increases vine susceptibility to winter injury and pathogens. | Veraison until harvest |
| Vine growth | Problems with graft union establishment. | Vineyard establishment |
*These symptoms are subtle, may not always be observed, or may be caused by other factors, such as nutrition, disease or vineyard management problems.
Contact information
Department of Horticulture, Oregon State University
Oregon State University Extension Service
Laboratories and Consultants Serving Agriculture in the Pacific Northwest
Additional information
Fuchs, M.F. 2007. Grape Leafroll Disease. Cornell University and the New York State IPM Program.
Golino, D.A., S.T. Sim, R. Gill and A. Rowhani. 2002. California mealybugs can spread grapevine leafroll disease. California Agriculture Journal 56(6):196-201.
Golino, D.A., E. Weber, S. Sim and A. Rowhani. 2008. Leafroll disease is spreading rapidly in a Napa Valley vineyard. California Agriculture Journal 62(4):156-160.
California Cooperative Extension: Mealybugs in California Vineyards
University of California IPM Online
Walton, V.M., P.A. Skinkis, et al. Preventative Management of Vine Leafroll Virus and Its Vectors. Corvallis, Oregon: Oregon State University Extension Service. Forthcoming.